Abstract
Background:
Introduction: The aim of the study is to evaluate influence of genetic variability in some proinflammatory genes on efficacy of adalimumab therapy in patients of psoriasis and on psoriasis development in patients with other autoimmune diseases treated by different biologics.
Methods: In the study, three patients with biologics were compared: 11 patients with psoriasis with finished treatment with adalimumab (A), 14 patients with psoriasis successfully treated by adalimumab (B), and 12 patients with other diagnosis treated by biologics with development of psoriasis (group C, 1 patient with hidradenitis suppurativa, 4 patients with m. Crohn, 3 patients with ulcerative colitis, 3 cases with ankylosing spondylitis, 1 patient with rheumatoid arthritis). These patients were treated by different biologics. In all patients, several polymorphisms in proinflammatory genes (I/D ACE, MMP-2, MMP-9, and in MDR1) were examined.
Results: Significantly lower effect of adalimumab treatment in 3 months in the A group compared to B group was observed (P=0.009), more frequently in women. In A group, a significant association of I/D ACE genotype with PASI and BSA before onset of adalimumab treatment and with positive family history of psoriasis at the first-degree relatives (parents, children, siblings) with MMP-9 -1575 T/C genotype. In B group, a longer successful treatment by adalimumab in GG genotype carriers in TNF alpha – 308 A/G (P=0.03), better treatment effect in the TT genotype of MMP-2 -790T/G (p=0.04) and in the CC genotype of MMP- 9 -1575 T/C (P=0.04) were found. In the heterogeneous C group, duration of biologics treatment correlated with onset of psoriasis from the beginning of the therapy by biologics and was significantly associated with the MDR1 polymorphism.
Conclusion: Finally, it is possible to conclude that success of biologics therapy is associated with some germ line genotypes in especially proinflammatory genes. Psoriasis development as a consequence of biologics treatment in different autoimmune diseases seems to be related to MDR1 gene variability which can responsible for many pharmacokinetic and pharmacodynamics aspects of therapy.
Keywords: Psoriasis; ACE; MMP-2; MMP- 9; MDR-1; Genetics; Biologics
Introduction
Autoimmune disease, characterized by the host’s immune response against self-antigens, manifests in more than 100 different types and affects more than 10% of the world’s population, causing significant morbidity. Psoriasis is amongst the most common autoimmune diseases affecting approximately 2% of people globally. The disease is a result of the net outcome of genetic and environmental factors whose multilevel interaction is not fully understood yet. Intense research efforts to date, had focused on examining these factors in isolation, resulting in hundreds of genetic risk loci identified, many of which are shared between the members of the autoimmune disease spectrum, such as psoriatic arthritis, Crohn´s disease and others. This genetic overlap seems to be an indication of overlap of common causal pathways [1]. The therapies for psoriasis are mainly symptomatic and sometimes with poor response. Response among patients is very variable. This variability may be partly explained by the effect of different genetic backgrounds. On the other hand, clinical manifestation of psoriasis can be developed during biologics therapy in patients with other autoimmunity diseases as a result of shared etiopathogenetic background of these diseases.
Using pharmacodynamics and pharmacokinetics approach,
pharmacogenetics and, of course, pharmacogenomics with the
whole genome knowledge, is able to describe the inheritance
influence on organism response to different drugs as well as
on the effect of dose, velocity of starting of effect, on different
time of elimination of drug from the body and drug interactions.
Necessity of precious diagnosis is of the greatest importance,
because different pato biochemical mechanisms can lead to similar
phenotypic characteristics of the disease. Individual response
on therapy can be modified by gene polymorphisms and/or
even rare alleles in those genes which directly or indirectly enter
the interactions with effect and/or metabolism of drug [1,2].
Pharmacogenetics and pharmacogenomics are being used also
to search for biomarkers that can predict response to systemic
treatments, including those for moderate-to-severe psoriasis.
Diverse systemic and biologic therapies are used to treat moderateto-
severe psoriasis. Moderate-to-severe psoriasis is usually treated
with systemic immunomodulators such as acitretin, cyclosporine,
and methotrexate.
Anti-tumor necrosis factor (TNF) drugs (adalimumab,
etanercept, or infliximab) are the first-line treatment for patients
resistant to conventional systemic therapies. Adalimumab was
initially approved in 2002 for the treatment of rheumatoid
arthritis. It has subsequently been approved for >10 additional
indications, including moderate to severe plaque psoriasis [3].
Because psoriasis has commonly higher prevalence in many other
diseases with autoimmune features, biologics therapy potentially
can improve or deteriorate clinical manifestation of psoriasis. This
could be individually influenced by gene polymorphisms forming
genetic predisposition to chronic inflammatory diseases. Although
these therapies are very efficient, around 30-50% of patients have
inadequate response. Ustekinumab is a monoclonal antibody that
targets interleukin (IL)-12 and IL-23 and is used for moderate-tosevere
psoriasis. New drugs (apremilast, brodalumab, guselkumab,
ixekizumab, and secukinumab) have recently been approved
for psoriasis. However, response rates to systemic treatments
for moderate-to-severe psoriasis range from 35 to 80%, so it is
necessary to identify non-invasive biomarkers that could help
predict treatment outcomes of these therapies and individualize
care for patients with psoriasis. These biomarkers could improve
patient quality of life and reduce health costs and potential side
effects [1,2]. However, very few pharmacogenetic studies have
examined the relationship between germline gene polymorphisms
and the response to biologicals [3,4]. Aim of the pharmacogenetic
pilot study was to compare three different groups of patients
with biologics treatment in genotype distributions and/ or allelic
frequencies of some germline polymorphisms in gene participating
on immunomodulation during chronic inflammatory state [5].
Material and Methods
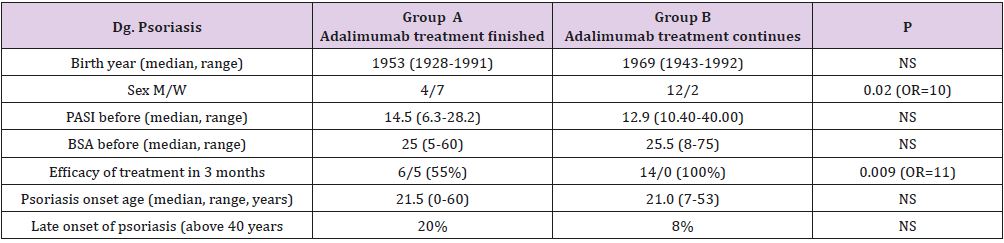
a) Group A: 11 psoriatic patients treated by adalimumab for
three months when the treatment was stopped due to insufficient
efficacy of disease (Table 1)
b) Group B: 14 psoriatic patients with efficient continuous
therapy by adalimumab (Table 1)
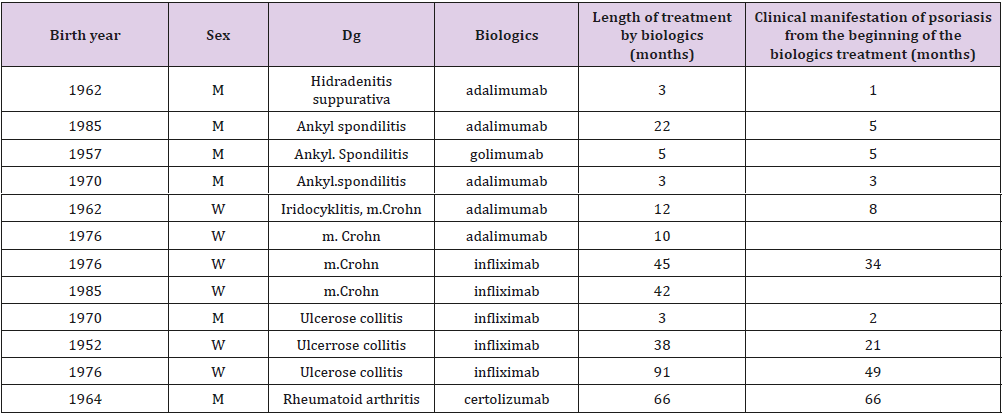
c) Group C: 12 patients with other than psoriatic diagnosis (1 case of hidradenitis suppurativa, 4 cases of m. Crohn, 3 cases of ulcerative colitis, 3 cases of ankylosing spondylitis, 1 case of rheumatoid arthritis, Table 2) in which clinical manifestation of psoriasis developed during different biologics therapy. Genotypes in several germline genetic polymorphisms - I/D ACE (rs4646994), TNF alpha -308 G/A (rs1800629), MMP-2 -690 T/G (rs243864) and MDR 1 C3435T (rs1045642) were identified by the PCR method and restriction analysis according to standard protocols.
Results
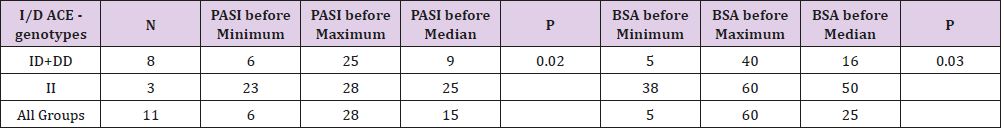
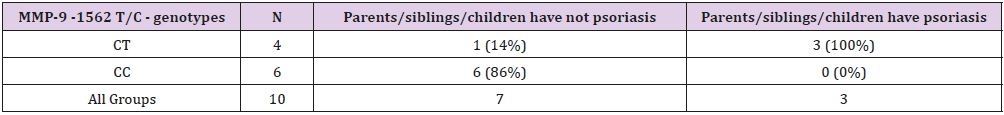
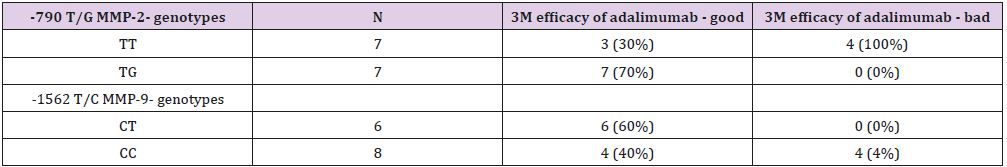
A significantly lower 3 months’ effect of adalimumab therapy in A compared to B group was confirmed (A group - 6 positive response to 5 negative responses to group B-14 positive to 0 negative; P=0.009; Table 1). In the A group, a significant association of II genotype in I/D ACE polymorphism and higher value of PASI and BSA markers before starting of adalimumab therapy was found (P=0.02; P=0.03, respectively; Table 3).A positive family history of psoriasis in the 1st heredity line (parents, siblings, children), was associated with genotypes in -1562 C/T MMP-9 (P=0.03; sensitivity 1, specificity 0.86 for CT genotype; Table 4) A significant association of duration of biologic treatment with genotypes of TNF- α – 308 A/G was found (P=0.03). The GA genotype carrier was treated significantly longer (median 35 months, range 17-87 months) compared to genotype GG carrier (median 15, range 2-30 months). The three months (3 M) effects of biological therapy differ between carriers of genotype TG and TT of MMP-2 -790T/G (sensitivity 0.7 and specificity 1 for TG, P=0.04).
Table 3: Differences in genotype distribution in I/D ACE polymorphism in PASI and BSA score before adalimumb therapy onset in Group A.
Table 4: Differences in genotype distribution in -1562 T/C MMP-9 polymorhisms in family history of psoriasis in Group A.
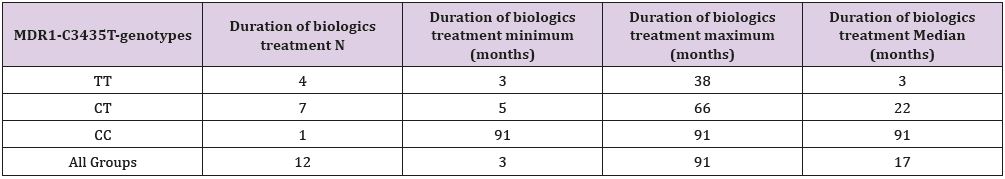
Similarly, the CT genotype carriers in MMP9-1562 C/T polymorphism have more frequently good response to adalimumab therapy in 3M period compared to TT genotype carriers (sensitivity 0.6, specificity 1.0; P=0.07; Table 5) In the C group, duration of biological therapy highly significantly correlated with the time from the beginning of the biological therapy until psoriasis became clinically manifest (P=0.00002). Duration of biological treatment was also significantly associated with C3435T MDR1 genotype (P=0.02, Table 6).
Table 5: Differences in genotype distribution in -790 T/G MMP-2 and -1562 T/C MMP-9 polymorhisms in family history of psoriasis in Group B.
Table 6: Differences in genotype distribution in -790 T/G MMP-2 and -1562 T/C MMP-9 polymorhisms in family history of psoriasis in Group B.
Discussion
Drug survival of biologics represents their real-world
effectiveness and safety. When a meta-analysis of real-world
evidence on the drug survival of biologics in treating psoriasis had
been performed, 37 studies with 32,631 subjects were included. The
drug survival for all biologics decreased with time, dropping from
66% at year 1 to 41% at year 4 for etanercept, from 69% to 47%
for adalimumab, from 61% to 42% for infliximab, and from 82% to
56% for ustekinumab [6]. Certainly, individual genome variability
must be to some extent responsible for these results. But, so far,
pharmacogenetic studies in psoriasis have generated divergent
results. Replication of findings in larger cohorts is required [7]
Our pilot study tries to accent the importance of knowledge of
interindividual variability in the main inflammatory genes which
can substantially influence all pharmacological aspects of systemic
therapy of psoriasis. According to our results, an association of
duration of biologic treatment with genotypes of TNF- α – 308
A/G was found. Patients with another polymorphic TNF-α-238 GG
genotype treated by biologics more frequently achieved a PASI75 at
6 months and those with the TNF-α-857CT/TT genotypes showed
greater improvements in PASI score and BSA and more frequently
achieved PASI75 in the study of Gallo et all. [5]
Several studies evaluated changing levels of TNF-α and MMP-9
in tissue and/or serum of psoriasis patients treated with biologics
but without genetic variability of susceptible genes [6,7]. We can
conclude that efficacy of biological treatment is associated with some
germ cell (innate) genotypes in genes contributing to inflammation
and wound healing (TNF alpha, matrix metalloproteinases).
Besides duration of treatment, clinical manifestation of psoriasis
in patients with other autoimmune diagnosis treated by biologics
seems to be associated with genotype in MDR-1 gene. Our pilot
results support necessity of evaluation of interindividual genome
variability in clinical studies which can support further introducing
of personalized medicine during development of new drugs for
(not only) psoriasis treatment [8,9].
References
- Vasilopoulos Y (2017) Pharmacogenetics and psoriasis: is targeted treatment a possibility? Pharmacogenomics 18(18): 1627-1630.
- Ovejero Benito MC, Muñoz Aceituno E, Reolid A, Saiz Rodríguez M, Abad Santos F, et al. (2018) Pharmacogenetics and Pharmacogenomics in Moderate-to-Severe Psoriasis. Am J Clin Dermatol 19(2): 209-222.
- Strober B, Crowley J, Langley RG, Gordon K, Menter A, et al. (2018) Systematic review of the real-world evidence of adalimumab safety in psoriasis registries. J Eur Acad Dermatol Venereol 32(12): 2126-2133.
- Linares Pineda TM, Cañadas Garre M, Sánchez Pozo A, Calleja Hernández MÁ (2016) Gene polymorphisms as predictors of response to biological therapies in psoriasis patients. Pharmacol Res 113(A): 71-80.
- Gallo E, Cabaleiro T, Román M, Solano López G, Abad Santos F, et al. (2013) The relationship between tumour necrosis factor (TNF)-α promoter and IL12B/IL-23R genes polymorphisms and the efficacy of anti-TNF-α therapy in psoriasis: a case-control study.Br J Dermatol. 169(4): 819-829.
- Lin PT, Wang SH, Chi CC (2018) Drug survival of biologics in treating psoriasis: a meta-analysis of real-world evidence. Sci Rep 8(1): 16068.
- van Vugt LJ, van den Reek JMPA, Coenen MJH, de Jong EMGJ (2018) A systematic review of pharmacogenetic studies on the response to biologics in patients with psoriasis. Br J Dermatol 178(1): 86-94.
- Mastroianni A, Minutilli E, Mussi A, Bordignon V, Trento E, et al. (2005) Cytokine profiles during infliximab monotherapy in psoriatic arthritis. Br J Dermatol 153(3): 531-536.
- Cordiali Fei P, Trento E, D Agosto G, Bordignon V, Mussi A, et al. (2006) Decreased levels of metalloproteinase-9 and angiogenic factors in skin lesions of patients with psoriatic arthritis after therapy with anti-TNF-alpha. J Autoimmune Dis 3: 5.

 Research Article
Research Article