Abstract
This study depicts the biodegradation as a strategy to eliminate the complex “adsorbent-adsorbate” obtained from adsorption process. The treatment of these secondary pollutants is very important not only because of its possible toxicity, but also due to the landscape alteration by its confinement. The purpose of this study was to investigate the simultaneous biodegradation of a complex of Brilliant Blue R-250 (BBR) and Sorghum vulgare straw, from a previous process of dye adsorption by the straw; the complex used was a load of 20 mg/g. Two fungi with capabilities to degrade BBR (C1) and S. vulgare (H1) were selected from a strain collection previously isolated from soils. The results showed the biodegradation of organic material through cellulolytic activity and the biodegradation of BBR and/or lignin by laccase, LiP and MnP enzymatic activities. Moreover, the results of absorbance spectra showed decrease in the intensity of the residual dye and aromatic compounds, and infrared spectra showed variation in different spectral regions demonstrating the alteration of bond of dye molecule and organic material by biodegradation process. The fungi were identified as Fusarium oxysporum y Fusarium graminearum by partial 18S rDNA sequencing and morphology.
Keywords: Adsorption complex; Brilliant Blue R-250; Biodegradation; Enzymatic Activities; Sorghum Vulgare Straw
Abbreviations:BBR: Brilliant blue R-250; LiP: Lignin Peroxidase; MnP: Manganese Peroxidase
Introduction
The rapid industrialization and urbanization in the world, has generated a large number of environmental issues; one of the most severe is the discharge of effluents containing high concentrations of dyes due to industrial processes carried out by the industries of food, cosmetic, plastic, textile and paper industries, among others. Pearce et al. [1] estimated that over 100,000 different types of dyes are in existence and the annual global production of synthetic dyes stands at over 700,000 metric tons. Hubbe et al. [2] reported that approximately 10-15%, (greater than 70,000 tons per year) of the dyes are potentially discharged into the environment. Colored effluent from dye consuming industries contains metabolites that confer undesirable properties to water streams and reservoirs where they pose serious risks to human health due to their toxicity, carcinogenicity, mutagenic and teratogenic effects [3,4].
Among the type of methods widely used for the removal of dyes is the adsorption which is a physicochemical method, being practical and efficient and offering great potential in the treatment of effluents. In recent years, lignocellulose materials such as agricultural waste and other industries have proved to be a good alternative as inexpensive bio sorbents. However, these removal processes may become a serious problem since the contaminant remains chemically intact, being it only adsorbed on the adsorbent. Thus, it is important to find processes that can eliminate both the dye and the bio adsorbent; this is where the bioprospecting of having microorganisms with metabolic capacity to carry out this task is important. The present work shows the simultaneous biodegradation of the complex “S. vulgare straw-Brilliant Blue R-250”, derived from an adsorption.
The sorghum has a composition of 6.03% of soluble compounds, cellulose 59.73%, 26.06% hemicellulose and 17.8% of lignin. A great variety of fungi and bacteria can digest these macromolecules using a battery of hydrolytic or oxidative enzymes; however, in native substrates, the binding of polymers hinders their biodegradation. Molecular genetics of cellulose-, hemicellulose- and lignin-degrading systems advanced considerably during the 1990s, and much is known about the structure, genomic organization, and regulation of the genes encoding these proteins [5]. Meanwhile, BBR a triphenylmethane dye, is also known as Acid Blue 83, Acid Brilliant Blue and Brilliant Indocyanine 6B, it was originally developed for dyeing wool, silk and nylon in the textile industry and later was used extensively in the painting, ink, plastic and leather industries, also for staining proteins in electrophoresis techniques, as well as for measuring protein concentrations. Regarding to biodegradation of this dye, little is known, it has only being reported that in presence of peroxidase enzyme modified with periodate dextran from radish, a decolorization of up to 80% to 90% occurs after 5 minutes of reaction [6]. Therefore, the biodegradation study of this contaminant is of interest as well as evidencing the feasibility of eliminating at the same time the biomaterial used as an adsorbent.
Material and Methods
Bio-Adsorbent Used
S. vulgare straw was acquired from forage of the town; it was used as adsorbent of BBR and the samples used had a load of 20 mg of BBR/g of straw.
Screening of Microorganisms
Initially the screening of microorganisms capable of decolorize BBR and/or produce cellulolytic activity was carried out using a total of 43 axenic microorganisms previously isolated from samples soil and the native microbiota from S. vulgare. The presence of cellulolytic enzyme was tested by the sting plates technique with colloidal cellulose as carbon source at 1% in mineral media containing in g/L 0.3 KNO3, 0.03 FeCl3.6H2O, 0.2 MgSO4.7H2O, 1 KH2PO4 and 0.1 CaCl2; after incubation time at 28°C for 10 days; the presence of hydrolysis halos was evaluated. The screening of microorganisms capable of decolorize BBR was made using the sting plate technique, containing mineral media, BBR at 100 mg/L and yeast extract at 0.03%. The total number of microorganisms were planted by sting and incubated, after the incubation time halos of decolorization were observed.
Biodegradation of BBR and S Vulgare
The selected fungal on the screening phase were evaluated to degrade S. vulgare straw and to decolorize BBR in liquid phase. Firstly, systems containing liquid medium supplemented with 0.03% yeast extract and BBR at 100 mg/L were prepared, they were inoculated with an agar cylinder of 0.5 cm diameter containing mycelial growth of each fungus previously selected. After incubation time the residual dye was quantified by measuring absorbance at 555 nm versus a calibration curve. Controls without inoculum were prepared and analyzed in the same way as the samples. For the degradation of S. vulgare straw, systems were also prepared containing 25 mL of mineral medium and 0.25 g of S. vulgare straw as carbon source. The systems were inoculated in the same way and then, they were incubated at 28°C and 180 rpm for 28 days. Every 7 days cellulolytic activity was evaluated through quantification of reducing sugar [7].
Biodegradation of the Complex “S. Vulgare -BBR”
Two series of systems containing 50 mL of mineral medium added of 1.0 g of S. vulgare straw with a dye load of 20 mg/g previously adsorbed were prepared. Fungal denominated C1 and H1 were inoculated in order to biodegrade S. vulgare straw and BBR, respectively. These fungal were used under two strategies; first, with both fungal simultaneously inoculated at the beginning of the process and second at different times (fungus H1 inoculated at the beginning process and C1 inoculated after 21 days), the systems were incubated at 28 °C, at 180 rpm for 56 days. Controls systems were prepared in the same manner without inoculum. The effect of adsorption of dye on S. vulgare was also evaluated over the simultaneous biodegradation process; for this, systems containing 50 ml of mineral media added of 1g of non-adsorbed S. vulgare were prepared; then fungal H1 and C1 were inoculated and the BBR was added at 20 mg/g. During the incubation period, cellulolytic activity, lignin peroxidase, manganese peroxidase and laccase activity were evaluated. Additionally, the absorbance spectrum of supernatant free cell from 200 to 800 nm was performed on a Thermo Scientific Genesys 10S spectrophotometer UV-Vis, also the supernatants were analyzed by infrared spectrometry FTIR on a Nicolet IS50 from 4000 to 650 cm-1 using 32 scans. The residual straw was quantified by gravimetric method. Finally, the two selected fungal were identified by partial sequencing. For this, total DNA was extracted from axenic cultures of each strain and partial 18S rDNA was amplified using the primers FwdV-18S (5’-AACGAAAGTTAGGGGATCG-3’) and RevV200-18S (5’-CCACCCACAAAATCAAGAAAGAGCTC-3’) which recognizes a region of 295 bp from 456465 to 456760 of Saccharomyces cerevisiae S288c chromosome XII, complete sequence GenBank Accesion NC_001144.5. The 295 bp rDNA PCR products were sequenced using the BigDye Terminator Kit v3.1 (Applied Biosystems Cat. 4636917 Foster City, California, USA). Final sequences were analyzed using Vector NTI Advance v11.0.
Similarity searches were performed using BLAST program v2.2.14 from NCBI (http://www.ncbi.nlm.nih.gov/). The partial 18S rRNA gene sequences were deposited in the GenBank Database under Accession Numbers KT225575.1 and KT225576.1. The evolutionary history was inferred using the Neighbor-Joining method [8]. The evolutionary distances were computed using the Maximum Composite Likelihood method [9] and evolutionary analyses were conducted in MEGA5 software [10].
Results and Discussion
Screening for Microorganisms
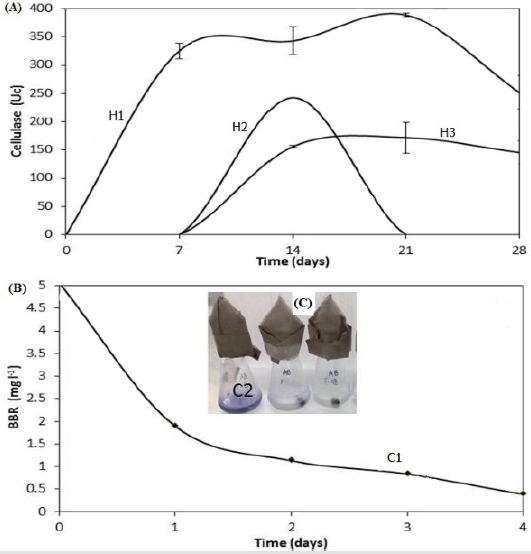
The screening searching for microorganisms with cellulolytic activity and/or decolorization BBR capacity showed that 10 of 43 had capacity to degrade cellulose. We selected the three that showed the largest halo of hydrolysis on solid medium, these were fungi which were denominated H1, H2 and H3. Regarding qualitative BBR biodegradation, it was found that only 2 of the 43 microorganisms showed the ability to make decolorization halos and to pigment their mycelium; these fungi were designated as C1 and C2. The ability of the selected fungi from the screening was explored to use S. vulgare straw as sole carbon source. Figure 1a shows that fungus H1(Fusarium oxysporum) showed the highest cellulolytic activity (388.6 units); this activity was detected at the initial time and lasted up to 21 days of incubation; in contrast, the enzyme activity of H2 and H3 isolates began after 7 days of incubation, showing a maximum activity of 242.4 and 171.7 units, respectively.
It has been found that genus Fusarium includes pathogenic species that cause many diseases, especially by means of the ability to invade and colonize plants due in part to the breakdown of plant barriers through ligno-cellulosic enzymes [11]. Also, Fusarium sp. secretes a number of hydrolytic enzymes capable of degrading cell wall polymers in order to invade the plant tissue; some of them have been detected as virulence factors for phytopathogenic process, such as pectic enzymes [12,13]. Little is known about specific biodegradation of S. vulgare, only authors have reported its hydrolysis by Trichoderma harzianu, to produce ethanol [14] and the use of S. vulgare seeds to produce reducing sugars [15].
With regard to the results of BBR decolorization in liquid medium it showed that both microorganisms selected were capable to decolorize the dye; C1 (Fusarium graminearum) showed a decrease of 62.0 % during the first day of incubation, and a total of 92.3 % after 4 days (Figure 1b). Meanwhile, the C2 fungus showed complete removal of BBR in a period of 24 hours, it apparently due to an adsorption process (Figure 1c). Based on these results, the H1 and the C1 strains were selected for their ability to degrade the straw and the BBR. Figure 2 shows the phylogenetic tree of their sequences and the evolutionary history was related to F. oxysporum and F. graminearum. The selected fungal were tested under the two strategies previously detailed to evaluate the biodegradation of the adsorbed dye and simultaneously the adsorbent.
Figure 1:
a) Cellulase activity of H1, H2 and H3 fungal using straw of S. vulgare as sole carbon source
b) BBR residual used as sole carbon source by C1 and C2 isolates previously selected in screening process.
Figure 2: Evolutionary relationships of taxa. The evolutionary history was inferred using the Neighbor-Joining method [8]. The tree is drawn to scale, with branch lengths in the same units as those of the evolutionary distances used to infer the phylogenetic tree. The evolutionary distances were computed using the Maximum Composite Likelihood method [9] and are in the units of the number of base substitutions per site. Evolutionary analyses were conducted in MEGA5 [10].
Biodegradation of the Complex “S. Vulgare-BBR”
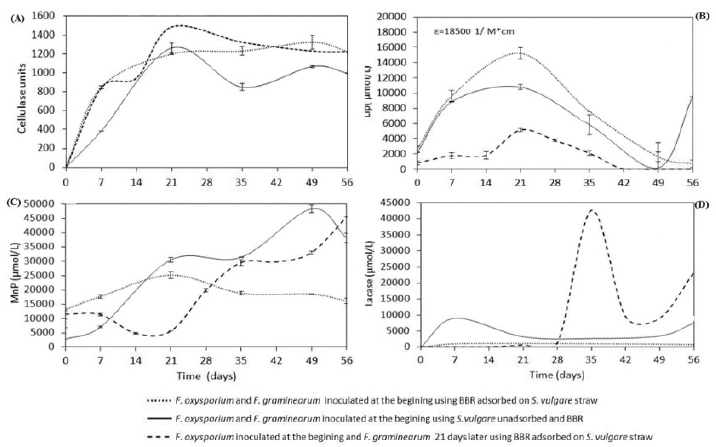
The first step to evaluate the biotic degradation of BBR and S. vulgare straw was by enzymatic activities measurement. The Figure 3a shows the results of the cellulolytic activity; where is clear that the maximum activity was at day 21 of incubation in the three kinetics, suggesting a comparable degree of biodegradation of S. vulgare straw using either strategy; that is, both fungus played an independently role on the substrates, where apparently the fungus F. graminearum, which is capable of degrading the dye, is not involved on straw biodegradation. Additionally, it is noted that the dye adsorption does not affect the biodegradation of S. vulgare straw.
Additionally, LiP, MnP and Lacasse activities were quantified; these enzymes catalyze the degradation of a wide variety of organic pollutants, such as dyes [16,17]; carbamazepine, diclofenac among others [18,19], also, these enzymes are involved in the degradation of lignin [20]. The Figure 3b shows the LiP activity, the three kinetic showed the highest activity at 21 days of incubation observing a decrease later on, this activity was higher when both fungi were inoculated at the beginning comparing with the strategy of inoculation at different times, it because this enzyme could metabolize S. vulgare and BBR simultaneously, and the strategy of single F. oxysporum inoculation during the first 21 days produced less activity, since this fungus mostly degrades S. vulgare. The detection of this enzyme during the kinetics is indicative of the biodegradation of lignin and lignocellulose and it is a good result, because these compounds are the most abundant in the used organic waste. According to Zhao et al. [21], lignin slows down biodegradation of cellulose and hemicellulose in lignocellulosic substrates because it acts as a physical barrier protecting the carbohydrates, therefore, lignin degradation can be considered as a key process during composting of lignocellulosic substrates. Additionally, as is shown in Figure 3b, the effect of dye adsorption over LiP activity, apparently enhanced its activity; this result could be due to the fact that adsorbed dye has greater physical contact with the fungal.
On the other hand, MnP activity was lower when using the strategy of inoculating both fungi at the beginning, compared with the activity quantified in the systems inoculated at different times. The fungus F. oxysporum apparently did not produce MnP activity during the first 21 days of incubation, and it was until inoculation of F. graminearum, when the enzyme activity was detected reaching levels equal to those obtained without adsorption (Figure 3c); this latter strategy suggests that adsorption limited the MnP activity. This same behavior occurred with the laccase activity (Figure 3d), showing that the kinetic using adsorbed BBR on S. vulgare showed the lowest activity (1,200 μmol/L) at day 7, when compared with the kinetic of non-adsorbed dye, where enzyme activity was up to 10,000 μmol/L at day 7. Furthermore, when fungal inoculation was at different times the MnP activity was detected from day 28, a week after the inoculation of F. graminearum; this is indicative its metabolism. These enzymes are involved in the biodegradation of BBR, Saraswathy et al. [16] reported the decolorization of BBR through an adsorption process and the laccase activity of Aspergillus tamarii, Penicillium purpurogenum and mixed cultures of Trichoderma sp and Aspergillus flavus, where they obtained 90% levels of BBR biodegradation.
Figure 3:
a) Cellulase activity
b) Lignin peroxidase activity
c) Manganese peroxidase activity and
d) Lacasse activity
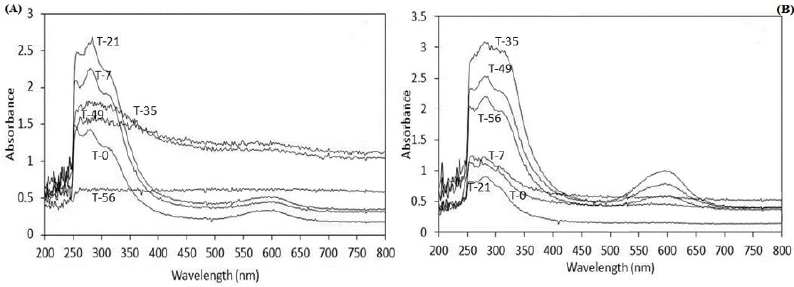
Moreover, the spectrophotometry analysis was another way to demonstrate the biodegradation. The results showed changes in the UV-Vis region, suggesting consumption and/or metabolite production during biodegradation process. Figure 4a shows the spectra from the system using both fungal at the same time with dye adsorbed on S. vulgare; at initial time (T-0) was detected a maximum absorbance between 240 to 360 nm corresponding to UV region, where aromatics compounds from dye and possibly aromatics of lignin from sorghum are responsible for this absorption; also there is a slight absorbance between 530 and 630 nm, a zone which absorbs the BBR at neutral pH. Although the dye was adsorbed on S. vulgare straw, a little part of it was released during the preparation process due to sterilization. After 7 and 21 days of incubation (T-7 and T-21), the absorbance between 240 and 360 nm was increased, which can be attributed to the release of a high content of aromatics from the biodegradation of the dye and/or lignin.
Also it shows that the absorbance between 530 to 630 nm was decreased, this possible attributed to consumption of the dye initially released; this occurred even when simultaneously there must have been release of dye due to the biodegradation of S. vulgare straw, this suggest that the dye and straw were simultaneously biodegraded. After 35 days of incubation the regions of maximum absorbance were diminished, suggesting that the aromatic compounds released into the supernatant were also degraded, achieving after 56 days no detection in these regions. The spectra corresponding to system where the fungal were inoculated at different times of incubation showed no variation during the first 21 days (Figure 4b), indicating that F. oxysporium was not participation individually; starting from the inoculum of F. graminearum (T-28), the behavior of increase in the region from 240 to 360 nm was observed, suggesting the degradation of BBR and S. vulgare.
Figure 4:
a) Absorbance spectra using F. oxysporum and F. graminearum at the beginning time of incubation with dye adsorbed on S. vulgare
b) Absorbance spectra using F. oxysporum during the first 21 days and both fungal the rest of the time.
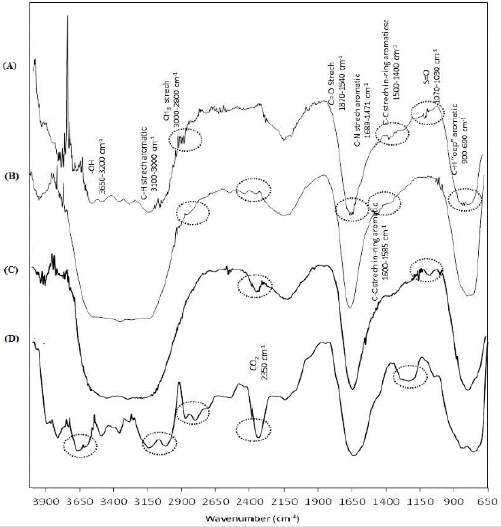
Additionally, molecular variations caused by biodegradation were analyzed by FTIR; Figure 5 shows the spectra at the beginning and the end kinetics. The regions detected at initial time (Figure 5a) were: -OH groups (constituent of culture medium) that absorbs from 3650-3200 cm-1, C-H stretch aromatic (3100-3000 cm-1); bonds of CH3 in region 3000-2800 cm-1; C-C stretch in-ring aromatic (1600-1585 cm-1) and (1500-1400 cm-1); bonds of S=O (1070-1030 cm-1); C-N stretch aromatic amines (1689-1471 cm-1) and finally C-H “opp” aromatic (900-690 cm-1), all of them detected in the BBR molecule. Furthermore, C=O stretch (1870-1540 cm-1) were observed, it localized in lignin and hemicellulose, probably released during sterilization process. During the biodegradation process some modifications in spectra can be observed; most of them show intensity changes, suggesting a variation in concentration. The Figure 5b shows the spectrum after 56 days of incubation when both fungal were inoculated at the initial time; the CH3 group located in the region of 3000-2800 cm-1 was absent at the end of the kinetic, and it is a functional group which is in the periphery of the lignin molecule and is more exposed to microbial biodegradation. Also, a new band located at 2350 cm-1 was observed, it corresponded to CO2, possibly dissolved in the culture medium from biodegradation of biopolymer and dye.
Meanwhile, Figure 5c shows the infrared spectrum using the fungal inoculated at different times; the profiles of bands are very similar to those described above. However, the disappearance of the bands located in the region of 3100-3000 cm-1, which belongs to stretch aromatic C-H, and a decrease in intensity of two unidentified bands located at 2368 and 2169 cm-1 were detected. Additionally, the infrared analysis of the kinetic using non-adsorbed BBR (Figure 5d) shows the bands described above, which showed different intensities related to qualitative concentration of the functional groups. The CH3 group was no detected after 56 days of incubation (3000-2800 cm-1). Furthermore, new bands can be seen such as that the ones located between 1310 and 1150 cm-1 which corresponds to C-N and C-O bonds, respectively; and 2350 cm-1 corresponding to CO2. Also, it showed a higher definition in the region 3100-3000 cm-1 corresponding to aromatic tension C-H and a modification in the area corresponding to -OH bond (3200-3650 cm-1). Finally, the gravimetric quantification showed a higher weight loss in the system inoculated with both fungal at the beginning of incubation, resulting in a loss of 43% including biomass by fungal growth.
Figure 5: FTIR spectra, (A) at initial time, (B) after 56 days of incubation when F. oxysporum and F. graminearum were inoculated at the beginning time using dye adsorbed on S. vulgare, (C) after 56 days of incubation using F. oxysporum during the first 21 days and both fungal the rest of the time using dye adsorbed on S. vulgare and (D) after 56 days of incubation using F. oxysporum and F. graminearum at the beginning time using dye non-adsorbed on S. vulgare.
Conclusion
The use of microorganisms with specific enzymatic activities is a useful strategy to degrade secondary pollutants such as complex “adsorbent-adsorbate” from adsorption processes. In this case the use of F. graminearum and F. oxysporum achieved simultaneous biodegradation of S. vulgare straw used as bioadsorbent of BBR- 250. This activity is expressed through cellulolytic, LiP, MnP and lacase enzymes; also, the analysis of UV-Vis spectrophotometry and infrared spectrometry confirmed the biodegradation. So, this research can be applied in systems of agro-industrial waste biodegradation of cellulolytic previously used as adsorbents.
Acknowledgement
This research was concluded during a sabbatical leave of absence of Cervantes-Gonzalez E, according the regulations of Autonomous University of San Luis Potosí.
References
- Keine D, Walker JQ, Kennedy BK (2018) Development, Application and Results from a Precision-medicine Platform that Personalizes Multi-modal Treatment Plans for Mild Alzheimer's Disease and At-risk Individuals. Curr Aging Sci 11(3): 173-181.
- Kim J, Yoon H, Basak J, Kim J (2014) Apolipoprotein E in Synaptic Plasticity and Alzheimer’s Disease: Potential Cellular and Molecular Mechanisms. Mol Cells 37(11): 767-776.
- Cramer PE, Cirrito JR, Wesson DW, Lee CY, Karlo JC, et al. (2012) ApoE-directed therapeutics rapidly clear beta-amyloid and reverse deficits in AD mouse models. Science 335(6075): 1503-1506.
- Bond M, Rogers G, Peters J, Anderson R, Hoyle M, et al. (2012) The effectiveness and cost-effectiveness of donepezil, galantamine, rivastigmine and memantine for the treatment of Alzheimer's disease (review of Technology Appraisal No. 111): a systematic review and economic model. Health Technol Assess 16(21): 1-470.
- Hernandez CM, Gearhart DA, Parikh V, Hohnadel EJ, Davis LW, et al. (2006) Comparison of galantamine and donepezil for effects on nerve growth factor, cholinergic markers, and memory performance in aged rats. J Pharmacol Exp Ther 316(2): 679-694.
- Jiang X, Chen LL, Lan Z, Xiong F, Xu X, et al. (2019) Icariin Ameliorates Amyloid Pathologies by Maintaining Homeostasis of Autophagic Systems in Aβ1-42-Injected Rats. Neurochem Res 44(12): 2708-2722.
- Guo E, Hu Y, Du T, Zhu H, Chen L, et al. (2019) Effects of Picrasma quassioides and its active constituents on Alzheimer's disease in vitro and in vivo. Bioorg Chem 92: 103258.
- Xu PX, Wang SW, Yu XL, Su YJ, Wang T, et al. (2014) Rutin improves spatial memory in Alzheimer’s disease trans-genic mice by reduing Aβoligomer level and attenuating oxidative stress and neuroinflammation. Brhav Brain Res 3(264): 173-180.
- Yu L, Wang S, Chen X, Yang H, Li X, et al. (2015) Orientin alleviates cognitive deficits and oxidative stress in Aβ1-42 induced mouse model of Alzheimer’s disease. Life Sic 121: 104-109.
- Zhao B (2009) Natural antioxidants protect neurons in Alzheimer’s disease and Parkinson’s disease. Neurochem Res 34(4): 630-638.
- Zhang SJ, Xu TT, Li L, Xu YM, Qu ZL, et al. (2017) Bushen-Yizhi formula ameliorates cognitive dysfunction through SIRT1/ER stress pathway in SAMP8 mice. Oncotarget 8(30): 49338-49350.
- Pietrzik C, Behl C (2005) Concepts for the treatment of Alzheimer's disease:molecular mechanisms and clinical application. Int J Exp Pathol 86(3): 173-185.

 Research Article
Research Article