Abstract
Recently, a simple protocol, named Sponge Like Protocol (SLP) was introduced for the preparation of bacterial ghost cells. This protocol is based on determination of both, the minimum inhibition concentration (MIC) and the minimum growth concentration (MGC) of some chemical compounds, which can induce pore(s) formation in the target microbial cell membrane/wall. Fine tuning of the effective concentrations of these chemical compounds enables cell evacuation while maintaining its correct 3D structure. The intact surface antigens are still able to activate the immune system as proven by the previous studies. The current study is a further step to evacuate another cell type, the spores of oyster mushroom, for the first time. The serial dilution method was controlled by direct plate count method to determine the correct concentrations of both MIC and MGC. Both of the DNA and proteins, which were released from the evacuated spores, were determined spectrophotometrically at 260 and 280nm, respectively. The evacuated spores’ quality was determined using both light and electron microscopes. The results showed a typical ghost-spore particle structure that can be used in medicinal, environmental, biotechnological, and forensic applications. We recommend the application of the current protocol for preparing various ghost-spore particles from different fungal origins. This method could have a potential use in reducing fungal spore pollution.
Keywords:Oyster mushroom; Spore; Spore Ghost; Sponge Like Protocol
Abbreviations: SLP: Sponge Like Protocol; MIC: Minimum Inhibition Concentration; MGC: Minimum Growth Concentration; SLRP: Sponge Like Reduced Protocol
Introduction
Different types of edible mushrooms are produced worldwide using different cultivation systems. In most cases, the production process is performed in closed climatized champers. On the other hand, half-open sheds are also used. In the closed growing champers, mushrooms produce and accumulate a massive number of spores causing severe problems including the developing of respiratory complaints ‘mushroom workers’ lung’ [1-3]. The contact with the mushroom spores is the main claimed factor. The exposure to the mushroom spores causes irregular breathing, coughing, shivering, fever and myalgia, in addition to lung volume decrease and increase in the number of white blood cells.Pleurotusostreatus, pearl or tree oyster mushroom is an important edible mushroom. It has been cultivated in Germany for the first time during the World War I to be used as food[4-9]. Oyster mushroom is also used for myco-remediation. It can be cultivated on straw and other natural or synthetic media [10-12]. It is one of the white-rot woods-decay fungi. It is a saprotrophic fungus that acts as a primary decomposer of wood, especially deciduous trees [13-18].P. ostreatushas different names. It is sometimes referred to as the tree oyster mushroom or the grey oyster mushroom to differentiate it from other species in the genus. Oyster mushrooms are mainly cultivated in large clear polyethylene bags with buns of hay layered in the bags, and spawn sown between the layers.It is also used in soups and stews in a similar fashion to meat [19-21]. Oyster mushrooms contain small amounts of arabitol, a sugar alcohol, which may cause gastrointestinal upset in some people [22,23]. It causes hypersensitivity for individuals in direct contact with its spores particularly for long time [3,24,25].
Evacuating microbes from their cytoplasmic content is a natural phenomenon [26]. Amara et al.[27]and others reported that pores could be introduced to the microbial cell walls and/or membranes as a result of different mechanisms [26,28-31], such as evacuating the gram-negative bacteria via bacteriophage infection [26]. Bacteriophage E lysis gene is used for evacuating the cells and turning them into ghost cells via controlling their expression using heat sensitive promoter [26,28,31-34]. Recently, the Sponge Like Protocol was introduced [27,35,36]. Its main concept is using active chemical compounds that can induce pores in the microbial cell membrane/wall and degrade its DNA at concentrations that do not change the surface antigens or the 3D structure [35]. This enables the evacuation of each of the gram-negative, gram-positive and spore forming bacteria, eukaryotes such as the yeast, as well as viruses such as Newcastle virus [26,35-48]. Recently, both Aspergillus flavus, Aspergillus niger, and their spores were evacuated and turned into ghost-spores [49,50].
SDS was proved to have the ability to perturb and destabilize cell wall/plasma membranes of both yeasts and fungi at very low concentrations [51]. SDS is also able to degrade the cell wall. H2O2 has oxidizing activity and able to degrade the genetic material instead of nucleases [38]. NaOH is also known for its damaging effect on microbial cell walls and a degrading capability for both DNA and RNA. It can deactivate microbial cells and can be used alone as antimicrobial agent as proved from the previous studies of microbial ghost cells preparations. NaHCO3 has a recognizable effect as salt and alkali as proved by Amara and Steinbüchel. It can deactivate various microbes or slow the growth of others in particular concentrations and for that it could be used in Spirulina platansisselective media (Amara and Steinbüchel. In this way, it has substituted CaCO3 in preparing ghost microbes other than Gram-negative bacteria. It might be useful to highlight that Amara and Steinbüchel have selected NaHCO3 based on a natural phenomenon that the Lake Chad becomes dominant with S. platensisduring the summer due to the evaporation of its water content and the increase in its salt content, where carbonate is the major constituent. Both of pH and salinity were responsible for eradicating various microbes and keeping the lake clean from microbes with an exception that S. platensiscould grow as a dominant microbe turning the colour of the lake to the cyanogreen colour [52]. In this study, for the first time, the spores of the oyster mushroom were evacuated from their content and turned into ghost particles using the concept of Sponge Like Protocol.
Materials and Methods
Isolation of the Oyster Mushroom
Mushroom fruiting bodies from Borg El-Arab region, Alexandria, Egypt, were collected during winter season and stored at 4oC. Under sterile conditions, small tissues of the wild fruiting bodies were transferred to the PDA plates. The mycelia tissue was placed in the center of the plate, so that growth can radiate away from it. The plates were incubated at 28oC for 7 days. After full colonization, the plates were stored at 4oC.
Morphological Identification
The fungal isolate was identified morphologically after growing on rice straw by observing its macroscopic features including: Cab and stem morphology, spore-print and microscopic shape of spores.
Oyster Mushroom Spawn Preparation
Spawn of the mushroom was prepared in 250 ml bottles using sorghum grains. The grains were washed with water thoroughly to clean the dirt, then boiled in water until being semi soft, after cooling to room temperature, the excess water was drained off and the grains were mixed with 5% (w/w) CaSO4. The bottles were filled to 3/4 with sorghum grains and sterilized by autoclaving at 121oC and 1.5 bar for 15 minutes. The sorghum grains were inoculated with actively growing mycelium of oyster mushroom from PDA plates and incubated at 28°C. Mycelial growth were allowed for 12 to 15 days until the mycelium fully covered the grains.
Oyster Mushroom Cultivation
Pleurotus sp.was cultivated using the perforated polythene bag methodwith minor modifications [53,54]. Dried rice straw was chopped into 5 to 7cm length and soaked in water for 4 hours in the presence of 5% (w/w) gypsum, and excess water was drained off. The substrate was sterilized by autoclaving at 121oC and 1.5 bar for 20min. About half kilogram of the substrate was placed in 40cm width X 60cm length polyethylene bags and spawned with 10% of the mushroom mycelia grown on sorghum grains. Spawning was done in 3 layers each above 5 cm layer of the rice straw substrate. The bags were subsequently placed into spawn running room at 25°C±2 under dark conditions. After the completion of spawn running, polythene bags were placed into the fructification room at 23°C±2 and 75–85% relative humidity. The bags were cut open on the sides without disturbing the bedsand sprayed twice a day with water for maintaining a high moisture level. The bags were exposed to light for 5-7 hours /day and left until the appearance of the mushroom pinheads. The fruiting bodies appear two weeks after bags perforation for 3-successive flushes. Mushroom was matured within 2-3 days after pinhead’s initiation. Mushroom was harvested by twisting the fruiting body to displace it from the base.
Collection of Oyster Mushroom Spores
The stems of the fresh oyster mushroom fruiting bodies were cut at the base of the fan-shaped cap. Then, the oyster mushroom cap was placed on a clean white paper with the underside down on the paper. A glass cup was placed over the mushroom cap and kept in a cool and dry place for 24 to 48 hrs. The glass cup and the mushroom cap were removed to find a spore print of the mushroom cap where the spores collected on the paper. Finally, the spores were collected in an Eppendorf tube by scrapping off the paper using sterile scalpel.
Determination of the MIC and MGC Concentrations for NaOH, SDS, NaHCO3 and H22 and Spore Viability
Standard broth microdilution susceptibility assay was used for determining the MIC values for each of NaOH, SDS, NaHCO3 and H2O2 [35,36]. Ten Eppendorf tubes, each contain 3mg of the spores in one ml of water were used. The different stocks of the used chemical compounds represent 10% of NaOH (non-autoclaved), SDS (autoclaved), NaHCO3 (autoclaved), and 30% of H2O2(commercial grade purchased from a pharmacy). After overnight incubation, direct plat counts were performed by spreading 100 µl of each spores-treated solution on PDA medium and the plates were incubated at 30oC for 5 days. The MIC value for each compound was calculated as well as the concentration that allowed first fungal growth detection (abbreviated as MGC) representing the concentration showing first growth after the MIC.
Determination of DNA /Protein Concentrations
The concentration of DNA in the supernatant, after each step for each randomization experiment, was determined spectrophotometrically by measuring the absorbance at 260/280nm using spectrophotometer (Biochrom LTD, Cambridge CB4 0FG, England). An extinction coefficient 260 = 1 corresponds to 50μg dsDNA mL-1 [54]. The DNA concentration was calculated automatically by the aid of the software of the spectrophotometer [55].
Spore Cells Evaluation Using Light Microscope
Spore cells generated from different treatments were investigated using light microscopy. The spores were stained on glass slides using crystal violet stain for 1 min, and then examined under light-microscope to check the evacuation process during various steps of the treatments.
Sample Preparation for Electron Microscope Examination
For further investigation of the quality of the ghost-spores, electron microscope was used to scan the spore particles. A spore smear of each preparation was prepared, and the smear surface was then coated with approximately 15nm gold layer (SPI-Module Sputter Coater).
Scanning of the Prepared Ghost-Spores (SGs) Surface
The gold-coated samples were scanned by analytical scanning electron microscope (Jeal JSM-6360, LA) with secondary element at 10kv acceleration voltage at room temperature. The digital images were then adjusted and analyzed.
Results and Discussion
Different groups of fungi are well known for their ability to produce spores as a part of their reproduction process and for protecting their origin. Such spores are usually more resistant to harsh environmental conditions and are able to spread everywhere. They are capable to reach our bodies particularly to our lungs. Some of them could cause severe illness for us, some cause allergy, while others if entered in small amounts might be safe. However, apparently, the exposure to safe spores for long time might cause different kind of illness [56]. Finding spore-less strains could be a good solution to this problem; however, establishing a protocol for deactivating the spores might be efficient to eliminate any kind of risk, particularly to the workers in mushroom production facilities. The size of some types of mushroom spores are quite bigger than other types of spore former fungi. Mushroom spores cause seasonal allergy for sensitive individuals. Deactivating such spores might be useful, while some types of spore-sensitivity are linked to the activity of the spores during their vegetation. In drug delivery system technology, empty mushrooms’ spores could be a good candidate, that could enrich different medicinal and biotechnological applications.
Mushrooms spores are invited candidates in forensic investigation, establishing a protocol for evacuating the mushroom spores using the concept of MIC will enable better realise to their DNA and protein contents. In some cases, the amount of the spores is too small and efficient protocol for isolating their DNA to be amplified by different PCR protocols are in demand. For those previous aspects, this study has been conducted to investigate the possibility of producing mushroom ghost-spores using the concept of the Sponge Like Protocol, which is based mainly on the determining of the MIC and MGC of some chemical compounds. These chemical compounds able to introduce pores into cell walls and membranes. Each of the Gram positive, Gram-negative, spore forming bacteria, yeast, fungi, and virus were turned into ghost cells using either SLP or the Sponge like Reduced Protocol (SLRP).The MIC for NaOH, SDS, and H2O2 was determined. In case of H2O2, the MIC was 1.11mL/mL from 30% H2O2. In case of SDS, MIC was 0.045mg/mL. Meanwhile, MIC of NaOH was 0.045mg/mL, and for NaHCO3the MIC was 3.7mg/mL.The MGC of NaOH, SDS, and H2O2was determined. In case of H2O2, the MIC was 0.37mL/mL from 30% H2O2. In case of SDS the MIC was 0.015mg/mL. In case of NaOH the MIC was 0.015mg/mL, and for NaHCO3 the MIC was 1.23mg/mL.
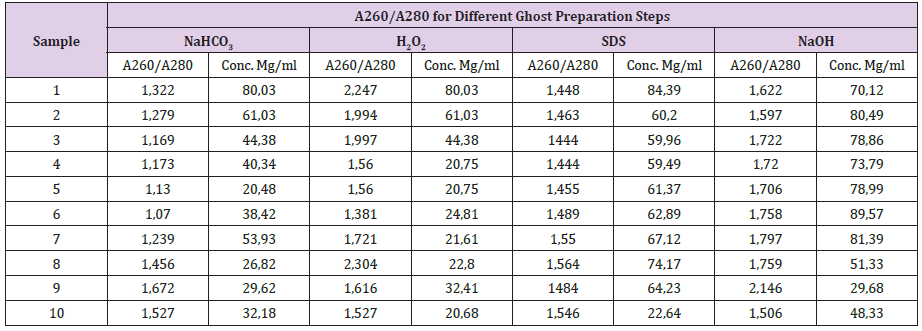
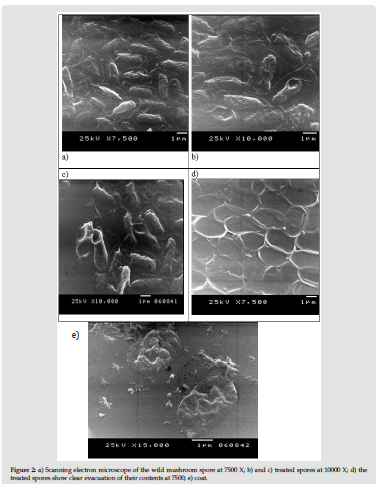
The unique part in this study is the investigation of the viability of the spores after each treatment during the serial dilution experiments using direct plate cultivation. The serial dilution method was done without the use of nutrients and only water was used. Using nutrients during the serial dilution experiment enable some microbes to develop some sort of defence mechanisms during the treatment as proved in previous studies. For example, the development of exopolysaccharide during K. pneumoniatreatment [56]. H2O2 is a potent agent in degrading both of DNA and RNA macro-molecules as proved by El Baky and Amara. As shown in Table 1 the different treatments showing successful release of both the DNA and protein. This has been confirmed by the images obtained from the electron microscope as images from a toe in Figure 1.And the protocol also succeeded to turn virus to ghost empty and inactive virus [38]. However, in order to prove the killing effect of different used compounds, direct cultivation on plates contain nutrients was done. This step gives better determination for the MIC and the MGC. Moreover, the usage of one ml of water and half ml of the chemical compound has led to narrowing the dilution differences, which give better MIC and MGC. In a previous study, we used 4.5ml of the medium and 0.5ml of the chemical compound, which give high dilution within the tubes. Both DNA and Protein percentages were calculated directly after the serial dilution experiment as in Table 1. The centrifugation, which happened only during collecting the spores from the treated samples to analysis them by either the electron or light microscopes, also help in evacuating the spores from their contents as in Figure 1&Figure 2 respectively.This study is an additional step for evacuating the different microbes as well as their components such as the spore of the mushrooms. Following the main concept of the cell evacuation protocol as described by Amara et al.[27]by using different chemical compounds after determining their minimum inhibition concentration will successfully lead to evacuating different biological materials and cells.
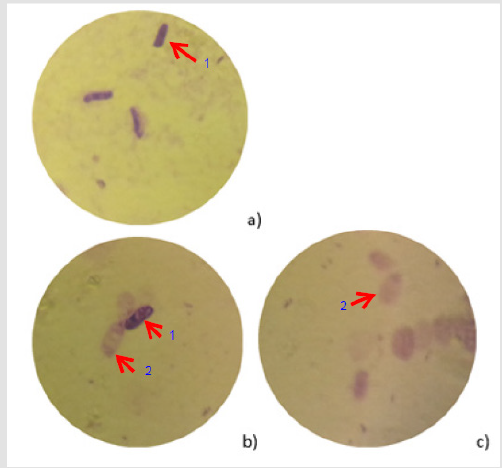
Figure 1: Different levels of the evacuation, spores (stained with crystal violeta)
(A) IgG,
(B) IgA, and
(C)a) Wild spore without treatment,
b) During the treatment processes (some of the spores still intact)
c) After treatment (all spores are evacuated) (Arrows: 1) non-evacuated spore; 2) evacuated spore).
Figure 2: a) Scanning electron microscope of the wild mushroom spore at 7500 X; b) and c) treated spores at 10000 X; d) the treated spores show clear evacuation of their contents at 7500; e) coat.
References
- Cox A, Folgering HT, van Griensven LZ (1988) Extrinsic allergic alveolitis caused by spores of the oyster mushroom Pleurotusostreatus. Eur Respir J 1(5): 466-488. <
- Noster U, Hausen BM, Felten G, Schulz(1976) [Mushroom worker's lung caused by inhalation of spores of the edible fungus pleurotus Florida ("oyster mushroom") (author's transl)]. Dtsch Med Wochenschr, 101(34): 1241-1245. <
- Horner WE, Ibanez MD, Liengswangwong V, Salvaggio JE, SLehrer SB (1988) Characterization of allergens from spores of the oyster mushroom, Pleurotusostreatus. J Allergy Clin Immunol 82(6): 978-986. <
- Bukhalo AS, Parkhomenko LP, Martinenko MM (1975) Growth of mycelium of Pleurotusostreatus (Fr.) Kummer in pure culture. MikrobiolZh 37(2): 181-184. <
- Obodai M, ClelandOkine J, Vowotor KA (2003) Comparative study on the growth and yield of Pleurotusostreatus mushroom on different lignocellulosic by-products. J Ind Microbiol Biotechnol 30(3): 146-149. <
- Tedesco G, Marchi A, Gerola FM (1983) Immunological study on the wall proteins of different fruiting portions in Pleurotusostreatus (Jacq. ex Fr.) Kummer and Agaricusbisporus (Lge.) Sing. G BatteriolVirol Immunol 76(7-12): 200-206. <
- Aggelis G, Iconomou D, Christou M, Bokas D, Kotzailias S, et al. (2003) Phenolic removal in a model olive oil mill wastewater using Pleurotusostreatus in bioreactor cultures and biological evaluation of the process. Water Res37(16): 3897-3904. <
- Alarcon J, Aguila S (2006) Lovastatin production by Pleurotusostreatus: effects of the C:N ratio. Z Naturforsch C 61(1-2): 95-98. <
- AssiJA, King AJ (2007) Assessment of selected antioxidants in tomato pomace subsequent to treatment with the edible oyster mushroom, Pleurotusostreatus, under solid-state fermentation. J Agric Food Chem55(22): 9095-9098. <
- Bhattacharya S, Das A, Prashanthi K, Palaniswamy M,Angayarkanni JMycoremediation of Benzo[α]pyrene by Pleurotusostreatus in the presence of heavy metals and mediators. 3 Biotech4(2): 205-211. <
- Migliore L, Fiori M, Spadoni A, Galli E (2012) Biodegradation of oxytetracycline by Pleurotusostreatus mycelium: a mycoremediation technique. J Hazard Mater 215-216: 227-232. <
- Vaseem H, Singh VK, Singh MP(2017) Heavy metal pollution due to coal washery effluent and its decontamination using a macrofungus, Pleurotusostreatus. Ecotoxicol Environ Saf145: 42-49. <
- Eichlerova I, Homolka L, Nerud F, Zadrazil F, Baldrian P, et al. (2000) Screening of Pleurotusostreatus isolates for their ligninolytic properties during cultivation on natural substrates. Biodegradation 11(5): 279-287. <
- Rigas F,Dritsa V, Marchant R, Papadopoulou K, Avramides EJet al. (2005) Biodegradation of lindane by Pleurotusostreatus via central composite design. Environ Int 31(2): 191-196. <
- Verma P, Madamwar D (2002) Production of ligninolytic enzymes for dye decolorization by cocultivation of white-rot fungi Pleurotusostreatus and phanerochaetechrysosporium under solid-state fermentation. Appl BiochemBiotechnol 102-103(1-6): 109-118. <
- WojtasWasilewskaM, Trojanowski J (1975) Studies on the decomposition of lignosulfonates by the fungi Pleurotusostreatus and Trametespubescens. Acta Microbiol Pol B 7(2): 77-90. <
- da Luz JM, Paes SA, Bazzolli DM, Totola MR, Demuner AJ,et al.(2014) Abiotic and biotic degradation of oxo-biodegradable plastic bags by Pleurotusostreatus. PLoS One 9(11). <
- Rosado FR, Germano S, Carbonero ER, Da Costa SM, Iacomini M, et al. (2003) Biomass and exopolysaccharide production in submerged cultures of Pleurotusostreatoroseus and Pleurotusostreatus "florida" (Jack.: Fr.) Kummer. J Basic Microbiol 43(3): 230-237. <
- Alam N, Amin R, Khan A, Ara I, Shim MJ, et al. (2008) Nutritional Analysis of Cultivated Mushrooms in Bangladesh - Pleurotusostreatus, Pleurotussajor-caju, Pleurotusflorida and Calocybeindica. Mycobiology36(4): 228-232. <
- Asada Y, Watanabe A, Irie T, Nakayama T, Kuwahara M (1995) Structures of genomic and complementary DNAs coding for Pleurotusostreatus manganese (II) peroxidase. Biochim Biophys Acta1251(2): 205-209. <
- AssiJA, King AJ (2008) Manganese amendment and Pleurotusostreatus treatment to convert tomato pomace for inclusion in poultry feed. Poult Sci 87(9): 1889-1896. <
- Adams S, Che D, Hailong J, Zhao B, Rui H, et al. (2019) Effects of pulverized oyster mushroom (Pleurotusostreatus) on diarrhea incidence, growth performance, immunity, and microbial composition in piglets. J Sci Food Agric99(7): 3616-3627. <
- Zhong L, Ma N, Wu Y, Zhao L, Ma G, et al. (2019) Gastrointestinal fate and antioxidation of beta-carotene emulsion prepared by oat protein isolate-Pleurotusostreatus beta-glucan conjugate. CarbohydrPolym 221: 10-20. <
- Jesenak M, Hrubisko M, Majtan J, RennerovaZ, Banovcin P (2013) Anti-allergic effect of Pleuran (beta-glucan from Pleurotusostreatus) in children with recurrent respiratory tract infections. Phytother Res 28(3): 471-474. <
- Paulik S, Svrcek, Mojzisova J, Durove A, BenisekZ, et al.(1996) The immunomodulatory effect of the soluble fungal glucan (Pleurotusostreatus) on delayed hypersensitivity and phagocytic ability of blood leucocytes in mice. ZentralblVeterinarmed B 43(3): 129-135. <
- Amara AA (2016) Lysozymes, Proteinase K, Bacteriophage E Lysis Proteins, and some Compounds for Microbial ghosts Preparation: A Review and Food for Thought. SOJ Biochem 2(1): 1-16. <
- Amara AA, SalemBekhit MM, Alanazi FK (2013) Sponge-like: a new protocol for preparing bacterial ghosts. The Scientific World Journal. <
- Dong H, Han X, Bai H, He L, Liu L, et al. (2012) Mutation of lambdapL/pR-cI857 system for production of bacterial ghost in Escherichia coli. Sheng Wu Gong Cheng Xue Bao 28(12): 1423-1430. <
- Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227: 680-685. <
- Makino K, Yokoyama K, Kubota Y, Yutsudo CH, Kimura S, et al. (1999) Complete nucleotide sequence of the prophage VT2-Sakai carrying the verotoxin 2 genes of the enterohemorrhagic Escherichia coli O157: H7 derived from the Sakai outbreak. Genes & genetic systems 74(5): 227-239. <
- Panthel K, Jechlinger W, Matis A, Rohde M, Szostak M, et al. (2003) Generation of Helicobacter pylori ghosts by PhiX protein E-mediated inactivation and their evaluation as vaccine candidates. Infect Immun71(1): 109-116. <
- Hensel A, Huter V, Katinger A, Raza P, Strnistschie C, et al. (2000) Intramuscular immunization with genetically inactivated (ghosts) Actinobacilluspleuropneumoniaeserotype 9 protects pigs against homologous aerosol challenge and prevents carrier state. Vaccine18(26): 2945-2955. <
- Weibull C (1956) The nature of the ghosts obtained by lysozyme lysis of Bacillus megaterium. Exp Cell Res10(1): 214-221. <
- Witte A, Wanner G, Sulzner M, Lubitz W (1992) Dynamics of PhiX174 protein E-mediated lysis of Escherichia coli. Arch Microbiol157(4): 381-388. <
- Amara AA, SalemBekhit MM, AlanaziFK (2013) Sponge-like: a new protocol for preparing Bacterial Ghosts. TSWJ 23(89): 1013. <
- Amara AA (2015)Saccharomyces cerevisiae Ghosts Using the Sponge-Like Re-Reduced Protocol SOJ Biochemp. 1-4. <
- Amara AA, Neama AJ, Hussein A, Hashish EA, Sheweita SA (2014) Evaluation the surface antigen of the Salmonella typhimurium ATCC 14028 ghosts prepared by "SLRP". Scientific World Journal2014: 840863. <
- ElBakyNA, Amara AA (2014) Newcastle disease virus (LaSota strain) as a model for virus Ghosts preparation using H2O2 bio-critical concentration. International Science and Investigation journal 3(5): 38-50. <
- Vinod N, Oh S, Kim S, Choi CW, KimSC et al. (2014) chemically induced salmonella enteritidisghosts as anovel vaccine candidate against virulent challenge in arat model. Vaccine32(26): 3249-3255. <
- Amara AA (2015) Bacterial and Yeast Ghosts: coliand Saccharomyces cerevisiae preparation as drug delivery model ISIJ Biochemistry 4(7): 11-22. <
- Amara AA (2015)Kostenlos viral ghosts, bacterial ghosts’ microbial ghosts and more. Schuling Verlag – Germany. <
- Vinod N, Oh S, Park HJ, Koo JM, Choi CW, et al. (2015) Generation of a Novel Staphylococcus aureus Ghost Vaccine and Examination of Its Immunogenicity against Virulent Challenge in Rats. Infect Immun 83(7): 2957-2965. <
- Amara AA (2016) The critical activity for the cell all degrading enzymes: Could the use of the lysozyme for microbial ghost’s preparation establish emergance oral vacccination protocol? International Science and Invastigation Journal 5(2): 351-369. <
- Amara AA(2016) Vaccine against pathogens: A review and food for thought.SOJ Biochemistry 2(2): 1-20. <
- Hussain ZM, Amra AA (2016) Case-by-case study using antibiotic-EDTA combination to control Pseudomonas aeruginosa. PakJ Pharm. Sci19(3): 236-243. <
- Park HJ, OhS, VinodN, JiS, Noh HB, et al. (2016) Characterization of Chemically Induced Bacterial Ghosts (BGs) Using Sodium Hydroxide-Induced Vibrio parahaemolyticus Ghosts (VPGs). International Journal of Molecular Sciences 17(11): 1904. <
- Wu X, JuX, DuL, Yuan J, Wang L, et al. (2017) Production of Bacterial Ghosts from Gram-Positive Pathogen Listeria monocytogenes. Foodborne Pathogens and Disease 14(1): 1-7. <
- Menisy MM, Ghazy A, Sheweita S, Amara AA (2017)Klebsiella pneumoniae Ghosts as Vaccine Using Sponge Like Reduced Protoco. Cellular and Molecular Medicine 3(2): 8. <
- El Baky NAS, Amer MM, Kholef E, Hussain HR, Abdel Rahman MZ, et al. (2018) Protein and DNA Isolation from Aspergillus Niger as well as Ghost Cells Formation. SOJ Biochemistry 4(1): 1-7. <
- ElBaky NAS, Amer MM, Kholef E,Hussain HR, Abdel Rahman MZ, et al. (2018) The minimum inhibition and growth concentrations for controlling fungal infections as well as for ghost cells preparation: Aspargillus flavus as a model. Biomedical Journal of Scientific and Technical Research10(2): 1-5. <
- HMDelley PA (1999) Cell wall stress depolarizes cell growth via hyperactivation of RHO1. J Cell Biol 147(1): 163–174. <
- Amara AA, Steinbüchel A (2013) New medium for pharmaceutical grade Arthrospira. Int J Bacteriol 9: 1-9. <
- Nehal MELDeeb, Hala IELAdawi, Abeer EAbd EL wahab, Ahmed M Haddad, Hesham AEL Enshasy, et al. (2019)Modulation of NKG2D, KIR2DL and Cytokine Production by Pleurotusostreatus Glucan Enhances Natural Killer Cell Cytotoxicity Toward Cancer Cells. Frontiers in Cell and Developmental Biology 7. <
- Bano ZS, HC (1962) Studies in the cultivation of Pleurotus sp. on paddy straw. Food Sci 12: 363-368. <
- Sambrook EFFJ, Mainiatis T (1989) Molecular Cloning a Laboratory Manual (2nd Edn). Cold Spring Harbor Laboratory, Cold Spring Harbor, NY, USA. <
- Menisy MM, Hussein A, Ghazy A, Sheweita S, Amro Abd Al Fattah Amara(2017) Klebsiella pneumoniae ghosts as vaccine using sponge like reduced protocol. Cellular and Molecular Medicine 3(2): 11.
-
<

 Research Article
Research Article