Abstract
Common variable immunodeficiency (CVID) is the primary immunodeficiency
disease with unknown etiology, characterized by impaired antibody responses. In
addition to the defect in humoral immunity, the number and function of T cells may be
impared in patients with CVID. Regarding interleukin-2 (IL-2) is a fundamental role in
survival, proliferation, and development of T cells as the effector arm of cell-mediated
immunity, the aim of this study was whether the situation of the cellualr immune
system and level of IL-2 in CVID patients differ from healthy subjects. Peripheral blood
mononuclear cells were isolated from whole blood of 20 CVID and 10 healthy subjects.
The cells were cultured in the presence or absence of phytohemagglutinin (2.5 μl/ml)
at 37˚C. The culture supernatant was collected after 72 hours and the IL-2 level was
measured by Enzyme-linked immunoabsorbant assay (ELISA).
The clinical and laboratory characteristics of the patients were also studied. CVID
patients showed the reduction in the levels of IgG, IgA, and IgM compared to healthy
individuals ( P < 0.0001). The CD4+ cell, CD8+ cell, and CD19+ cell frequencies and
CD4/CD8+ cell ratio in patients were significantly reduced (P < 0.001-0.05). Stimulated
PBMCs of patients significantly produced lower level of IL-2 than healthy controls ( P
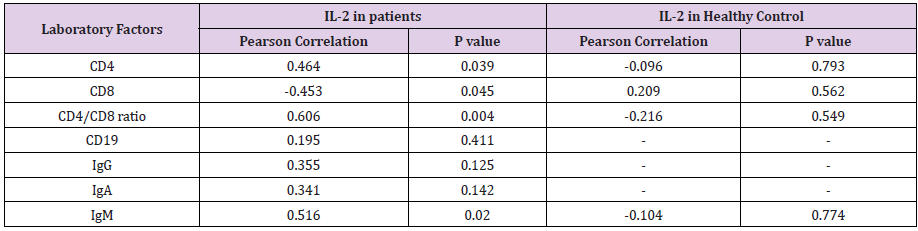
< 0.0001). IL-2 level in CVID patients was positively associated with Igm level, CD4 cell
frequency, CD4/CD8 cell ratio (P < 0.05, R = 0.464), while this association was negative
for CD8+ cell precentage. Based on these findings, IL-2 may be considered as a useful
drug supplement to treatment of infectious and non-infectious complications in CVID
patient through enhancing cellular immunity.
Keywords:Common Variable Immunodeficiency; Interleukin-2; Cell-Mediated Immunity
Abbreviations: CVID: Common Variable Immunodeficiency; ELISA: Enzyme-Linked Immunosorbent Assay; ESID: European Society for Immunodeficiency; PBMCs: Peripheral Blood Mononuclear Cells; SEM: Standard Error of Mean; PBS: Phosphate Buffered Saline
Introduction
Common variable immunodeficiency (CVID) is defined as a heterogenous disease which involves the production of antibodies, espetially immunoglobulin (Ig) types IgG, IgM, and IgA1. Although the etiology of CVID remains unknown, several genetic factors have been proposed for this disorder, including mutations in ICOS, CD19, BAFF-R, and TACI genes [1]. Patients with CVID mainly suffer from recurrent bacterial infections, autoimmunity, and malignancy, which contribute to a defect in humoral immunity such as the defect in differentiation of B lymphocyte into plasma cell and deficiency in antibody production [2]. Although the defect in humoral response is considered as the most important impairment, in some of CVID patients, the reduced number or impaired function of T lymphocytes have been observed [3,4]. Some CVID patients exhibit the reduction in circulating CD4+ T cells, particularly the cells of the CD4+.CD45RA+ subset [5]. Moreover, some patients with CVID indicate a profound defect in the proliferation of antigenstimulated T cell in vitro [6].
Extensive data from the literature have revealed that several molecular mechanisms may participate in the decreased number and impaired function of T cells [4,7,8]. One of the mechanisms involved in T cell defects is the reduced production or impaired fuction of cytokins such as IL-2, IL-12, and IL-4 which play the fundamental roles in T lymphocyte receptor signaling, B cell functions, and correlations between T and B lymphocytes [2,7,9]. Previous studies have shown that in some cases of CVID patients, B lymphocytes have an immature phenotype which produce antibody in the presence of factors such as IL-10, IL-4, IL-2, and co-stimulators [8]. On the other hand, 60 % of patients have low secretion of IL-2 upon T lymphocyte stimulation with mitogens owing to CD4+ T lymphocyte deficiency, which results from deficiency in bone marrow progenitor cells or defect in thymic maturation of T cells [3]. Regarding the fact that IL-2 has an indispensable role in the development and function of T cells, the aim of this study was whether the situation of cell-mediated immunity and level of IL-2 produced by stimulated PBMCs from Iranian patients with CVID differ from healthy subjects.
Materials and Methods
Study Populations
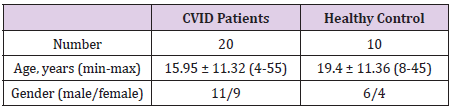
A total of 20 individuals were recruited among those referred to Allergy and Immunology Department of Children’s Medical Center, Kashan, Iran from January 2017 to July 2018 (Table 1). CVID was diagnosed by the specialist according to the European Society for immunodeficiency (ESID) criteria including: hypogammaglobulinemia (with a marked decrease of two out of IgG, IgA, and IgM isotypes at least two standard deviation (SD) below the mean of age and lack of isohemmagglutinin titer), onset of immunodeficiency at greater than two years of age, bacterial recurrent infections, a poor response to vaccination, and the rejection of other causes of humoral immunodeficiencies (hyper-IgM, SIgA, and X-linked agammaglobulinemia) [10,11]. The sampling was performed after 3-4 weeks of the last intravenous immunoglobulin (IVIG, 400 mg/kg) injection. None of CVID patients did not receive the drugs which influence the immune system and antibodies production (i.e. gold salts, phenytoin, sulfasalazine, steroids, and antimalarial drugs) at the time of the blood sampling. All of the patients were alive during the study. 10 healthy volunteers without any history of health or immunodeficiency problems were also participated as a control group. This study was approved by the Ethics Committee of Kashan University of Medical Sciences and preformed according to the declaration of Helsinki. All participants gave the informed consent before entering the study.
Table 1: The demographic characteristics of the CVID and healthy subjects.
Age are expressed as mean ± SD (range).
Peripheral Blood Mononuclear Cells (PBMCs) Isolation
Heparinized blood samples (3 ml) were obtained from CVID and healthy subjects. PBMCs were isolated using Ficoll density centrifugation according to the manufacturer’s instructions (Miltenyi Biotec, Germany). After centrifugation, the cells were collected from the interface between Ficoll and the plasma and washed several times with phosphate buffered saline (PBS). PBMCs were suspended in PBS and cell count was done with a haemocytometer. Cell viability was also measured using trypan blue dye exclusion.
Determination of the Immunologic Situation of Patients
To evaluate the humoral and cellular immune situations of patients and affirm CVID disease, the serum levels of IgG, IgA, and IgM in CVID patients and healthy volunteers were measured using an enzyme-linked immunoabsorbant assay (ELISA) kit according to the manufacturer’s guidlines (Mabtech, Sweden). The serum levels of IgG, IgA, and IgM in healthy individuals served as control group. Furthermore, the expressions of CD3, CD4, and CD19 on PBMCs of patient and healthy subjects were assessed using a FACSCalibur flow cytometry (Becton Dickinson, San Jose, CA). Accordingly, the cells were stained with fluorescein isothiocyanate (FITC) antihuman CD4 (eBioscience, USA), Phycoerythrin/Cyanine5 (PE/ cyn5) anti-human CD19 ( BioLegend, USA), and Phycoerythrin (PE) anti-human CD8 ( BioLegend, USA) antibodies or the matched isotype control IgG for 25 min at 4°C. The matched isotype control antibodies were used as negative controls. Afterwards, the cells were washed twice with PBS and the percentages of the stained cells were measured by a FACSCalibur system and then analyzed using FlowJo software (v10.1, FlowJo, Ashland, OR, USA).
PBMC Stimulation and IL-2 Measurement
The isolated cells were cultured in 96-well, flat-bottomed microtiter plates, at 37°C with 5% CO2. All assays were performed in duplicate. The cells were then stimulated with phytohemagglutinin (PHA, 2.5 μl/ml). PBMCs cultured without stimulation were considered as negative control. After 72-hour incubation, the supernatant of the cultures were collected and the level of IL-2 was measured using ELISA ( Mab Tech, Sweden) based on the manufacturer’s protocol.
Delayed-Type Hypersensitivity (DTH) Test
To evaluate the situation of cellular immunity in CVID patients in vivo, DTH test was carried out using purified protein derivative diphtheria, tetanus, and candida albicans antigens. The results of skin test less than 3 mm for all three antigens were determined as DTH anergy, while the results of 3 mm or greater for at least one antigen served as DTH positive.
Statistical Analysis
The results are presented as mean ± standard error of mean (SEM). Data analysis was done using GraphPad Prism 6 (GraphPad software, San Diego, CA). The comparison of two groups with normal and non-normal distribution were preformed using unpaired t-test and Mann-Whitney test, respectively. Spearman’s test was used to determine the correlation coefficients of the data with non-normal distribution and Pearson’s test in the case of normal distribution. P value < 0.05 was considered statistically significant.
Results
Description of Patients
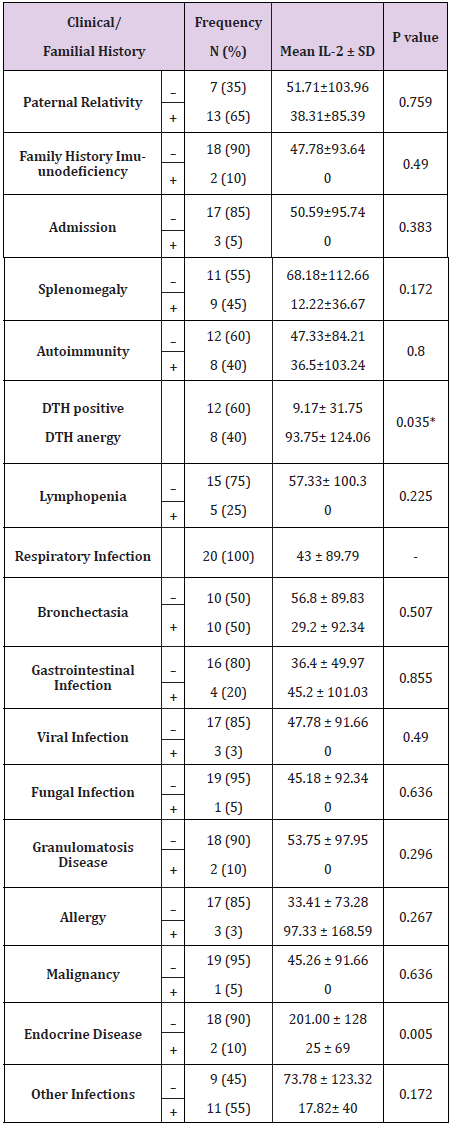
20 CVID subjects (aged 15.95 ± 11.32, mean ± standard deviation, range: 4 to 55 years) were paticipated in the study. The onset of clinical symptoms in patients was varied and occurred from an age of 3 years old to 18 (Table 2). The age in which the illness was diagnosed varied from a range of 2 years old to 19 (data not shown). The most frequent clinical manifestations among CVID patients were respiratory and gastrointestinal infections, bronchectasia, and splenomegaly (Table 2). All CVID patients had respiratory infections, which two had viral infections and one had fungal infections (Table 2). Of the 20 CVID patients, eight were DTH positive, 15 had gastrointestinal infections, ten had bronchectasia, nine had splenomegaly, eight had autoimmunity, five had lymphopenia, four had granulomatous disease (Table 2). Of all CVID patients, three CVID patients had allergy, thrombocytopenia; two had endocrine disease, autoimmune hepatitis, celiac disease, and hemolytic anemia; one had malignancy, myasthenia gravis, colitis ulcerative, and juvenile rheumatoid arthritis (Table 2). Table 2 is depicted the clinical characteristics of CVID subjects.
Table 2: Correlations of IL-2 level with the familial history and clinical characteristics of CVID patients.
Immunologic Features of CVID Patients
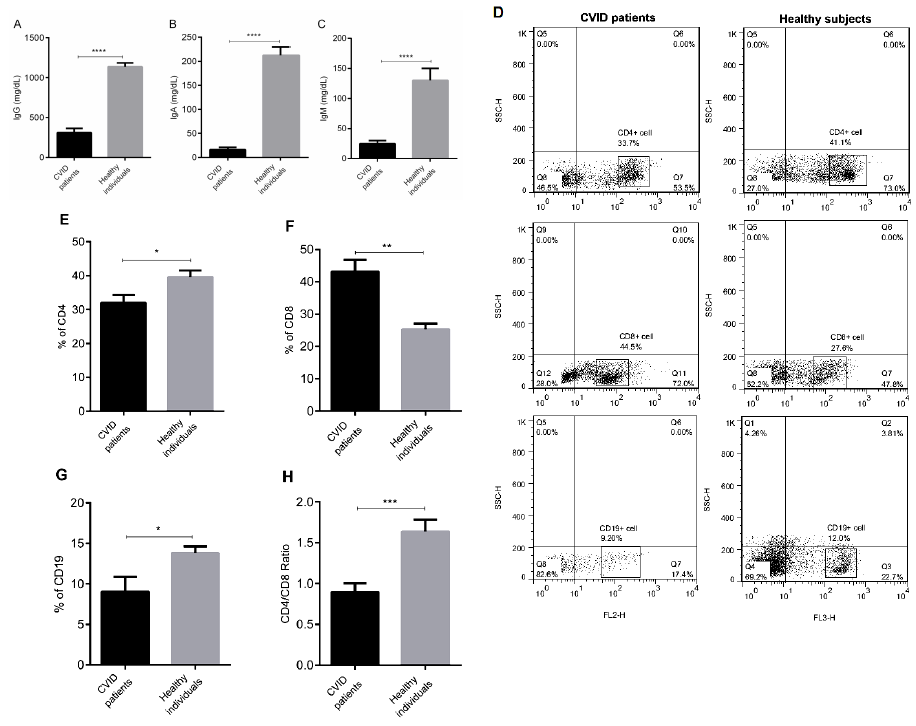
To determine the situations of humoral and cellular immunity in patients and confirm CVID disease, the serum levels of IgG, IgA, and IgM and frequencies of CD4+, CD8+, and CD19+ cells in CVID patients were assessed. As shown in Figures 1A-C, IgG, IgA, and IgM levels in the patients were significantly reduced compared to healthy control (P < 0.0001). The level of IgG in 50 % of patients was less than 400 mg/ml (data not shown). The same trend was observed in the percentages of CD4+ and CD19+ cells in CVID patients (Figures 1D, E & G, P < 0.01-0.05). On the contrary, CD8+ cell frequency was significantly higher in CVID patients than healthy controls (Figures 1D & F, P < 0.01). The proportion of CD4+/CD8+ cells revealed that CD4+/CD8+ cell ratio in CVID patients was significantly increased compared to control group (Figure 1H, P < 0.0001).
Figure 1: The characteristics of humoral and celluar immunity of CVID and control subjects. The levels of
(A) IgG,
(B) IgA, and
(C) IgM were measured by ELISA. The precentages of CD4+, CD8+, CD19+ and CD4+/CD8+ cell ratio were studied by flow
cytomerty (D-H).
The results are representative of 20 independent experiments for CVID group and ten independent experiments for healthy
individuals. Each bar in Figure.1 (A-H) shows mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
The Assessment of Cytokine Production of PBMCs Isolated from CVID Patients
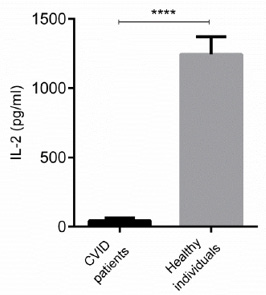
Regarding the fact that IL-2 participates in key functions of the immune system, especially cell-mediated immunity, IL-2 production of stimulated PBMCs from CVID and healthy subjects was studied. Our results indicated that PBMCs of CVID patients significantly produced lower level of IL-2 than control group (Figure 2, P < 0.0001). Furthermore, IL-2 production in 93.65% of CVID patients was less than 50 pg/ml (data not shown).
The levels of IL-2 in Patients with DTH Positive and Anergy
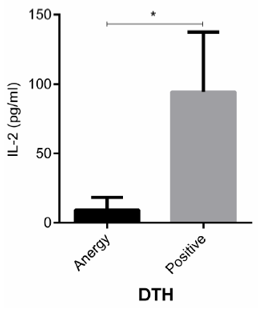
Regarding the fact that IL-2 is an important cytokine in cellmediated immunity, the levels of this cytokine was measured in patients who were DTH positive and anergy, which is an in vivo manifestation of cell-mediated immune response. Our data revealed that patients with DTH positive had significantly higher level of IL-2 than those who had anergy (Figure 3, P < 0.05). The correlation test indicated that IL-2 level in CVID patients was negatively associated with DTH positivity, as expected ( P < 0.05, Table 2).
Figure 2: IL-2 levels in CVID and control subjects. The level of IL-2 in CVID and healthy subjects were measured by ELISA. Data are representative of ten independent experiments for healthy subjects and 20 independent experiments for CVID group. Data are shown as mean ± SEM. ****p < 0.0001.
Figure 3: IL-2 levels in CVID patients with DTH positive and anergy. CVID patients were divided into two groups according to DTH positive and anergy. The level of IL-2 in CVID subjects was measured by ELISA. Data are representative of 20 independent experiments for CVID group and ten independent experiments for healthy subjects. Data are shown as mean ± SEM. *p < 0.05.

 Research Article
Research Article