Abstract
Summary: The aim of the study was to retrospectively evaluate our series of Testis Sparing Surgery (TSS) relating to the last 10 years and to discuss the feasibility, safety, and outcome of this treatment modality.
Patients and Methods: we here present a series of 10 pediatric patients ranging from 9 to 15 years (mean age 11.9yrs) referred to our Institution for mono lateral testicular lesion accidentally detected at US or for indefinite testicular pain and submitted to TSS. The lesions measured from 6 to 20mm (mean 13.1 mm). Histological diagnosis showed 2 residues of adrenocortical tissue,1 epidermoid cyst,3 Large cell calcifying Sertoli cell tumor, 2 Leydig cell tumors; 1 Leydig cell hypertrophia; 1 gonadoblastoma. Surgery was performed through an inguinal approach by cold ischemia and with organ-sparing surgery.
Results: Neither of the patients exhibited intraoperative or postoperative complications. After a mean follow-p of 6.7 years the patients were free of disease.
Conclusion: Testis-sparing surgery by cold ischemia and frozen-section examination is advisable for pediatric patients with benign testicular masses.
Keywords: Testis-Sparing Surgery; Children; Leydig Cell Tumor; Sertoli Cell Tumor
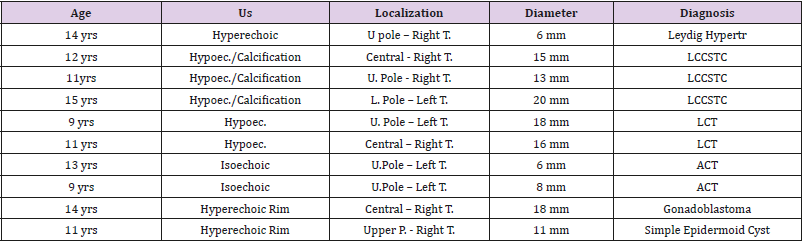
Abbreviations: US: Ultrasound; yrs: years; U: Upper; T: Testis; L: Lower; mm: millimeters; LCCSTC: Large Cell Calcifying Sertoli Cell Tumors; LCT: Leydig Cell Tumor; ACT: Adrenocortical Tissue
Short Communication
Until the 1980s, orchiectomy was generally considered the sole therapy fortesticular nodules, particularly in case of pediatric patients. However, there is actually a risk of overtreatment by radical orchiectomy, especially for clinically silent small lesions only detected by ultrasound (US) [1].The high level of accuracy achieved by frozen-section examination (FSE) for identification of both benign and malignant lesions, and the increasing attention paid to the cosmetic, functional, and psychological outcomes for young patients with testicular tumors strongly support an organsparing approach. The aim of this report was to retrospectively evaluate our series of Testis Sparing Surgery relating to the last 10 years and to discuss the feasibility, safety, and outcome of this treatment modality.
Patients and Methods
Before the surgery, all patients had undergone complete clinical and instrumental staging, including clinical examination, scrotal US, and computed tomography (CT) scans (abdominal and lung). Furthermore, the tumor markers alpha fetoprotein (AFP), betahuman chorionic gonadotropin (β-hCG), and lactate dehydrogenase (LDH) were evaluated to rule out a potential malignancy. Ultrasound was performed using high-frequency linear-array transducer (8–13 MHz) that allowed for high-resolution imaging of the testicles with a maximum lateral spatial resolution of 0.1 mm. Our case-series includes 10 patients, ranging from 9 to 15 years (mean age 11.9 years), who were referred to our Institution for mono lateral testicular lesions ranging from 6 to 20 mm in maximum diameter (mean 13.1 mm),presenting an indefinite testicular pain or an accidentally US-detected nodule. Histological diagnosis showed: 2 renmants of adrenocortical tissue (ACT), 3 Large cell calcifying Sertoli cell tumors, 2 Leydig cell tumors (LCT); 1 Leydig cell hypertrophia, 1 gonadoblastoma, 1 epidermoid cyst. Demographic data of the Series is showed in Table 1.
Surgery was performed through an inguinal approach by cold ischemia [2,3]. The testis and the surrounding tunica vaginalis were extracted from the scrotum. A soft clamp was applied at the highest level of the cord and the testis and spermatic cord were cooled by surrounding them with a frozen and granular sterile saline solution. The tunica vaginalis was then opened at the closest point to the tumor. Intraoperatively, the location of the tumor was determined by US, or by palpation (when possible). The lesions were isolated by incising the testis with a cuneiform resection of the nodule, and the material was sent for FSE. Additionally, biopsies were taken from the surrounding remaining tissue to verify that no solid tumor had been left behind and to confirm eventually testicular intraepithelial neoplasia. Once the pathologist report confirmed the diagnosis and the complete excision of the lesion, the parenchyma was sutured and the frozen saline solution was removed along with the clamp. A complete orchiectomy was performed in the case of gonadoblastoma. Follow-up consisted of clinical examination, abdominal and scrotal US performed every 6 months, and an annual chest X-ray in case of Leydig cell tumors. Urological follow-up was then scheduled every 6 months for two years, as well as annual chest X-ray if clinically indicated.
Results
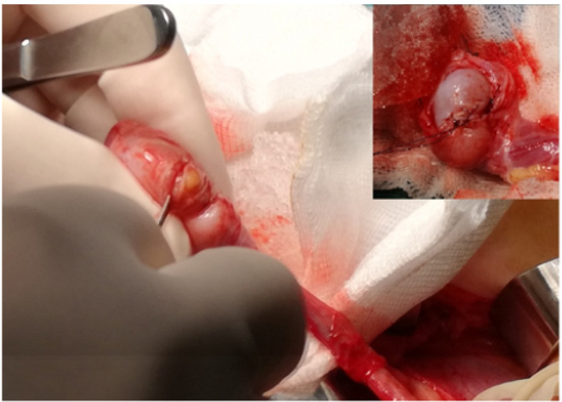
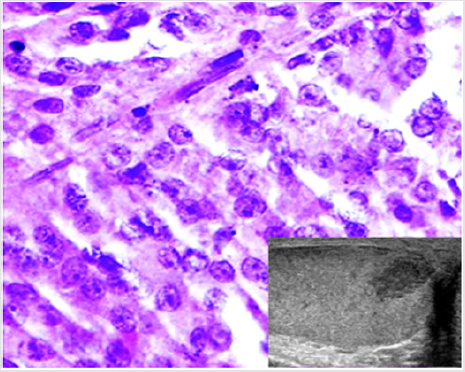
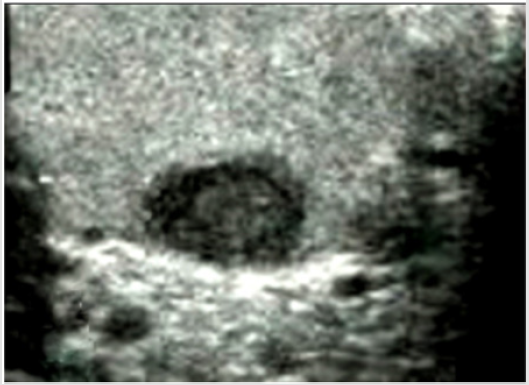
No significant intraoperative bleeding was observed. Neither perioperative infections nor hematomas occurred; no other perioperative or late complications were encountered. Definitive histology did not report any malignant histopathological features in either of the patients. The CT scans did not reveal abnormalities in either of the patients. All the patients were free of disease after a mean follow-up of 6.7 years (range 10-1) (Figures 1-3).
Figure 1: Intraoperative image showing the testis in contact with a frozen and granular sterile saline solution. The tunica vaginalis appears opened at the closest point to the tumor.
Figure 2: A case of Leydig cell tumor. Histological examination showing cells with abundant slightly eosinophilic cytoplasm, round nuclei and evident nucleoli (H&E, 40x). The neoplasm appeared as a hypoechoic nodule at US scan (inset).
Discussion
Testicular tumors are rare in childhood, and they are benign in 25% of cases [4]. In light of their uncommon occurrence at a young age, the initial data regarding testis-sparing surgery have been obtained from adult patients. Generally, testicular tumors with a size <25 mm in diameter are defined as small testicular masses, and masses with a diameter <20 mm can be considered good candidates for TSS because nearly 80% of these lesions are benign. Our caseseries shows a large array of pathologies confirming the crucial role of FSE to safely perform TSS [5]. Indeed, none of the preoperative tests can clarify the exact diagnosis. For this reason, all testicular masses should be assumed to be malignant, until proven otherwise. This is particularly true in pediatric cases, and informed consent should always include the possibility of an orchid funiculectomy. There is usually a dissection plane between healthy tissue and the tumor mass. Preoperative and sometimes intraoperative ultrasonography is extremely helpful and enables the surgical team to perform enucleation.
Once the frozen-section has confirmed that the tumor is benign and that the surgical borders are tumor-free, vascular occlusion should be terminated immediately and orchiopexy performed. In both of our cases, the results of frozen section and pathological examinations of paraffin blocks were identical. Some Authors have described warm ischemia conditions lasting less than 30 mins during the occlusion of vascular supply [6], and no testicular atrophy was reported in these cases. This is probably due to relatively shorter periods of ischemia. The development of late testicular atrophy has been reported in an experimental study, and some authors strongly recommend cold ischemia conditions [7,8]. In our opinion, cold ischemia is very useful for ensuring a wider safety margin for both the surgeon and the pathologist, for a possible deepening or to increase the resection margins. Testicular US was performed in all our cases, demonstrating high sensitivity, although MRI can be an efficient diagnostic tool for uncertain cases [9]. At present, a combination of preoperative evaluation and intraoperative biopsy could help urologists to distinguish testicular benign tumors and malignant tumors effectively.
In fact, it was feasible and reliable in this study as well as in recent reports, and no recurrence or atrophy was recorded. Moreover, preservation of the testis could reduce the physiological and psychological impact on patients. Therefore, a careful preoperative evaluation of pediatric testicular tumors, including scrotal US and serum markers [10], should be taken into consideration, and TSS should be offered to children with benign tumors if possible. In our opinion, the tumor size (<20 mm) and the volume of residual normal tissues (>1.0 cm) were important indicators for this procedure. Our results with this small group are consistent with the findings in the literature: the lesions were small (≤ 20mm) and negative for tumor markers [11,12]. The ideal follow-up interval after conservative treatment of benign testicular tumors is still not well defined. Patients with Leydig cell tumors, as well as complex epidermoid cysts, should be followed by medical monitoring, including a clinical examination and US, although the frequency of these medical evaluations and the duration are still debated Wegner et al. [13] found one local recurrence after conservative therapy and no progressive disease after reviewing 15 patients with Leydig cell tumors [14-17]. Interestingly, our Series includes 3 cases of LCCSCT. This is an exceptionally rare neoplasm originating from sperm cord cells [18]. The lesions may occur in an isolated form (approximately 60% of the cases) or in the context of a more complex genetic syndrome (approximately 40% of the cases) including Peutz-Jeghers syndrome and Carney complex [18]. For these reasons, after the diagnosis, a careful clinical evaluation of the patient is necessary to exclude a tumor with syndromic manifestation.
Conclusion
TSS represents a modern, safe and effective way to treat benign testicular lesions in children and adolescents with potential longterm psychological, cosmetic, and functional benefits. TSS should be used in pediatric patients with testicular masses in which the healthy testicular tissue appears to be salvageable based on US and FSE. Further prospective investigation is warranted to determine the oncologic outcomes of TSS for both pubertal and post pubertal patients.
References
- Giannarini G, Dieckmann KP, Albers P, Heidenreich A, Pizzocaro G (2010) Organ-sparing surgery for adult testicular tumours: a systematic review of the literature. Eur Urol 57(5): 780-790.
- Gierke CL, King BF, Bostwick DG, Choyke PL, Hattery RR, et al. (1994) Large cell calcifying Sertoli cell tumor of the testis: Appearance at sonography. AJR Am J Roentgenol 163(2): 373-375.
- Masur Y, Steffens J, Ziegler M, Remberger K (1996) Leydig cell tumors of the testis--clinical and morphologic aspects. Urologe A 35(6): 468-471.
- Ciftci AO, Bingöl Koloğlu M, Senocak ME, Tanyel FC, Büyükpamukçu M, et al. (2001) Testicular tumors in children. J Pediatr Surg 36(12): 1796-1801.
- Steiner H, Höltl L, Maneschg C, Berger AP, Rogatsch H, et al. (2003) Frozen section analysis-guided organ-sparing approach in testicular tumors: technique, feasibility and long-term results. Urology 62(3): 508-513.
- Emre S, Ozcan R, Elicevik M, Emir H, Soylet Y, et al. (2017) Testis sparing surgery for Leydig cell pathologies in children. J Pediatr Urol 13(1): 51-54.
- Miller DC, Peron SE, Keck RW, Kropp KA (1990) Effects of hypothermia on testicular ischemia. J Urol 143(5): 1046-1048.
- JS Valla for the Group D'Etude en Urologie Pédiatrique (2001) Testis-sparing surgery for benign testicular tumors in children. J Urol 165(6): 2280-2283.
- Cassidy FH, Ishioka KM, Mc Mahon CJ, Chu P, Sakamoto K, et al. (2010) MR imaging of scrotal tumors and pseudotumors. Radiographics 30(3): 665-683.
- Ronchi A, Pagliuca F, Franco R (2019) Testicular germ cell tumors: the changing role of the pathologist. Ann Transl Med 7(6): 204.
- Tetu B, Ro JY, Ayala AG (1991) Large cell calcifying Sertoli cell tumor of the testis. A clinicopathologic, immunohistochemical and ultrastructural study of two cases. Am J Clin Pathol 96(6): 717-722.
- Galosi AB, Fulvi P, Fabiani A, Servi L, Filosa A, et al. (2016) Testicular sparing surgery in small testis masses: A multinstitutional experience. Arch Ital Urol Androl 88(4): 320-324.
- Wegner HE, Dieckmann KP, Herbst H, Andresen R, Miller K, et al. (1997) Leydig cell tumor--comparison of results of radical and testis-sparing surgery in a single center. Urol Int 59(3): 170-173.
- Marte A (2018) Laparoscopic Finding of Ectopic Adrenocortical Tissue in a 2-Year-Old Boy with Vanishing Testis. European J Pediatr Surg Rep 6(1): 4-6.
- Ye YL, He QM, Zheng FF, Guo SJ, Zhou FJ, et al. (2017) Trends of testis-sparing surgery for pediatric testicular tumors in South China. BMC Surg 17(1): 31.
- Mennie N, King SK, Marulaiah M, Ferguson P, Heloury Y, et al. (2017) Leydig cell hyperplasia in children: Case series and review. J Pediatr Urol 13(2): 158-163.
- Anheuser P, Kranz J, Stolle E, Höflmayer D, Büscheck F, et al. (2019) Testicular epidermoid cysts: a reevaluation. BMC Urol 19(1): 52.
- Kaluzny A, Matuszewski M, Wojtylak S, Krajka K, Cichy W, et al. (2012) Organ-sparing surgery of the bilateral testicular large cell calcifying sertoli cell tumor in patient with atypical Peutz-Jeghers syndrome. Int Urol Nephrol 44(4): 1045-1048.

 Short Communication
Short Communication