Research Article
Combination of L-Carnitine and Angiotensin-II type
1 Receptor Blocker has Beneficial Effects on Hepatic
Fibrosis in a Non-Alcoholic Steatohepatitis Rat Model
Takuya Kubo, Hideto Kawaratani*, Yasuhiko Sawada, Yukihisa Fujinaga, Takahiro Ozutsumi, Daisuke
Kaya, Yuki Tsuji, Keisuke Nakanishi, Masanori Furukawa, Kou Kitagawa, Soichiro Saikawa, Shinya Sato,
Hiroaki Takaya, Kosuke Kaji, Naotaka Shimozato, Kei Moriya, Tadashi Namisaki, Takemi Akahane,
Akira Mitoro and Hitoshi Yoshiji
Author Affiliations
Department of Gastroenterology, Nara Medical University, Japan
Received: December 04, 2019 | Published: December 16, 2019
Corresponding author: Hideto Kawaratani, Department of Gastroenterology, Nara Medical University, Japan
DOI: 10.26717/BJSTR.2019.23.003969
Inflammation and oxidative stress contribute to the progression of nonalcoholic
steatohepatitis (NASH). Hepatic fibrosis and activated hepatic stellate cells (Ac-HSCs)
are attenuated by Angiotensin-II type 1 Receptor Blocker (ARB), and L-carnitine is
effective for NASH by ameliorating oxidative stress, but neither agent is effective in
a clinical setting. We evaluated the effect of the combination of L-carnitine and ARB
on liver fibrosis using a rat NASH model. A Choline-Deficient/L-Amino Acid-defined
(CDAA) diet was fed to F344 rats for 8 weeks. The rats were then divided into a control
group, group receiving L-carnitine or ARB alone, and group receiving L-carnitine plus
ARB. Therapeutic efficacy was assessed by evaluating liver fibrosis, liver fatty acid
metabolism, and oxidative stress. ARB inhibited liver-specific tumor necrotic factor-α
and LPS-binding protein, which are involved in hepatic inflammation. L-Carnitine
reduced hepatic oxidative stress by rescuing hepatic sterol-regulatory elementbinding
protein 1 and thiobarbituric acid reactive substances induced by the CDAA
diet. Combination of L-carnitine and ARB improved liver fibrosis, with concomitant
HSC suppression. Therefore, we suggest that L-carnitine and ARB are effective in
suppressing liver fibrosis. Currently both drugs are in clinical use, and a combination of
the two could be an effective therapy for NASH fibrosis.
Keywords: Angiotensin-2 type 1 Receptor Blocker; L-carnitine; Nonalcoholic
Steatohepatitis; Hepatic Fibrosis; Oxidative Stress
Non-Alcoholic Fatty Liver Disease (NAFLD) is the most common
liver disorder in developed countries. It is divided into simple fatty
liver and non-alcoholic steatohepatitis (NASH), which involves liver
inflammation. NASH is a progressive liver disease leading to hepatic
fibrosis, cirrhosis, and cancer, and it does not have an established
treatment. The pathogenesis of NASH is like that of alcoholic
steatohepatitis, with a two-step process beginning with excessive
fat accumulation in the liver, followed by aggravating factors such
as inflammatory cytokines, oxidative stress, and endotoxins [1].
However, it has also been suggested that inflammatory cytokines,
oxidative stress, endotoxins, and other factors are contributing
factors [2]. NAFLD progression is dependent on both genetic and
environmental factors [2,3], including non-synonymous Single-
Nucleotide Polymorphisms (SNPs) in PNPLA3 and TM6SF2 [4,5], as
well as oxidative stress and inflammation.
L-Carnitine (4-N-trimethylammonium-3-hydroxybutyric
acid), which is involved in β-oxidation of fatty acids, is a nutrient
conditionally synthesized from methionine and lysine in the brain,
liver, and kidneys, especially, the liver is the main tissue for carnitine
synthesis [6], and is mainly obtained from meat and dairy products
[7]. It is an accepted treatment for mitochondrial myopathy
and encephalomyopathy as well as other states of primary and secondary carnitine deficiencies [8] and has recently been applied
to treat hepatic encephalopathy.
On the contrary, the renin-angiotensin-aldosterone system
plays an important role in chronic liver disease [9,10]. We
previously reported that blocking angiotensin-II (AT-II) signaling
via the AT-II type 1 receptor (AT1R) suppresses liver fibrosis in rats
[11,12]. Furthermore, the inhibitory effect of Angiotensin-2 Type 1
Receptor Blocker (ARB) on hepatic fibrosis is consistent with the
suppression of activated hepatic stellate cells [13]. In addition, ARB
improves liver fibrosis via AT-II-mediated LPS-toll-like receptor 4
(TLR4) signaling and suppresses TLR4 signaling in Ac-HSCs [14].
We hypothesize that L-carnitine might improve the progression
of NASH by inhibiting oxidative stress, and its use in combination
with ARB may inhibit the development of NASH fibrosis via
various mechanisms. In this study, we examined the effect of the
combination of L-carnitine and ARB on NASH fibrosis using a rat
model fed a Choline-Deficient/L-Amino Acid-defined (CDAA) diet.
Animals and Regents
Male 6-week-old Fisher 344 (F344) rats were purchased from
Japan SLC (Hamamatsu, Shizuoka, Japan). L-Carnitine was purchased
from Otsuka Pharmaceutical Co. Ltd. (Tokyo, Japan). Losartan
was purchased from Merck Co., Ltd. (Tokyo, Japan). Conventional
chemical reagents were purchased from Funakoshi (Tokyo,
Japan). CDAA and choline-supplemented/L-amino acid-defined
(CSAA) diets were purchased from CLEA Japan Inc. (Tokyo, Japan).
Experimental Design
After one week of acclimatization, 30 rats weighing 160-175 g
were randomly divided into five groups. A control group that was
fed the CSAA diet for normal non-NASH baseline reference. Four
other groups were provided the CDAA diet to establish diet-induced
hepatic steatosis and fibrosis, which simulates human NASH. One
group received no further treatment, while the second group received
additional L-carnitine at 200 mg/kg/day and the third group
received the ARB losartan at 30 mg/kg/day. Finally, a combination
treatment group received both L-carnitine and losartan. These
treatment conditions were maintained for 8 weeks, during which
the rats had free access to tap water. At the end of the experimental
period, the rats were anesthetized with pentobarbital and sacrificed,
and the liver samples were collected. All animal procedures
were performed in accordance with the Declaration of Helsinki and
in compliance with the standard recommendations for the proper
care and use of laboratory animals. The protocol was approved by
the Animal Care and Use Committee of Nara Medical University.
Histological and Immunohistochemical Analyses
Five-micrometer-thick sections of formalin-fixed and paraffinembedded
liver specimens were stained using hematoxylin and eosin
and Azan stains to evaluate fibrosis. Besides, immunohistochemical
staining of alpha smooth muscle actin (α-SMA; DAKO, Kyoto, Japan),
which correlates with Ac-HSCs, was performed as previously
described [15,16]. The stained sections were analyzed using Adobe
Photoshop software ver. 6 (Adobe, Tokyo, Japan).
Quantitative RT-PCR Analysis
mRNA was extracted from frozen liver tissues using the RNeasy
Mini Kit (QIAGEN, Tokyo, Japan). The total RNA (1 μg) extracted
from each sample was reverse transcribed into cDNA using a high
capacity RNA-to-cDNA kit (Applied Biosystems Inc., Foster City, CA,
USA). As TNF-α correlates with hepatic inflammation, it leads to
liver fibrosis. As direct detection of LPS is difficult, we evaluated
hepatic LPS-binding protein (LBP) because this directly correlates
with LPS [17]. LPS stimulates TLR4 and activates Kupffer cells,
which leads to hepatic inflammation. Hepatic oxidative stress
was evaluated using sterol-regulatory element-binding protein 1
(SREBP-1) mRNA. The expression of mRNA encoding liver tissuederived
TNF-α, LBP, and SREBP1 was analyzed using the PowerUp
SYBR Green Master Mix and Step One Sequence Detection System
(Applied Biosystems Inc., Foster City, CA, USA) by qRT-PCR. The PCR
conditions were as follows: 95°C for 20 s, and 40 cycles at 95°C for
3 s and 60°C for 30 s. β-Actin was used as the endogenous control.
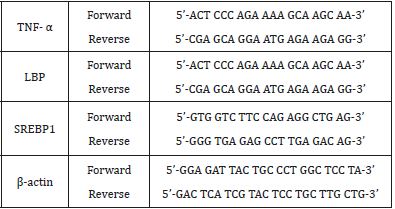
The sequence of primers used is shown in Table 1.
Measurement of Thiobarbituric Acid Reactive
Substances (TBARS)
We evaluated hepatic oxidative stress using hepatic TBARS
concentration. Liver homogenates were prepared with PBS using a
tissue homogenizer (Power Masher II®; Nippi, Tokyo, Japan), and
then protein was extracted. TBARS concentration was measured
using a commercially available kit, with malondialdehyde as the
standard (Cayman Chemical, MI, USA).
Statistical Analyses
All results are expressed as mean ± SD. Statistical analyses
were performed using EZR ver. 1.40 (Saitama Medical Center, Jichi
Medical University). All tests were two-tailed and the results with p
values < 0.05 were considered statistically significant.
Inhibitory Effect of L-Carnitine and ARB on Hepatic
Fibrosis
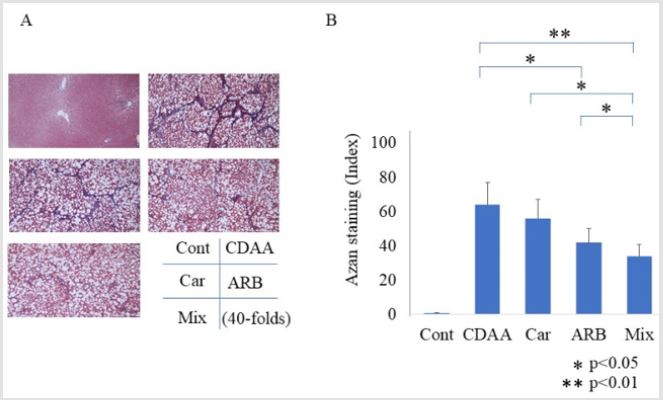
The groups fed the CDAA diet showed activated liver fibrosis
compared with that of the group fed the CSAA diet. In the L-carnitine
group, liver fibrosis was slightly improved compared with that in
the CDAA-only group (p = 0.07), and significantly improved in the
ARB group (p < 0.05). The combination of L-carnitine and ARB
resulted in a greater improvement than with either drug alone
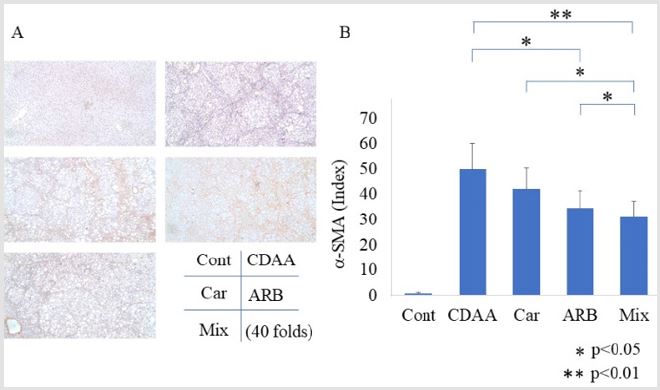
(Figure 1A & 1B). Immunohistochemistry of α-SMA indicated a
significant decrease in α-SMA-immunopositive Ac-HSCs in groups
treated with ARB (Figure 2A). The semiquantitative analysis of
α-SMA immunohistochemistry showed reduced α-SMA positive
staining along with the inhibition of hepatic fibrosis (Figure 2B). A
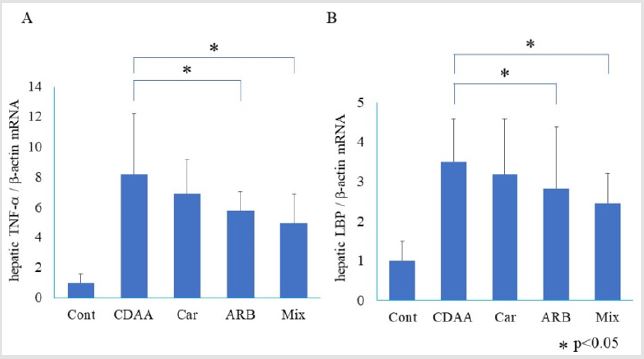
significant inhibition of TNF-α mRNA expression was also observed
in the ARB group compared with that in the CDAA group (Figure
3A) (p < 0.05). However, L-carnitine did not inhibit TNF-α mRNA
expression compared with that in the CDAA group. The combination
of L-carnitine and ARB resulted in an inhibitory effect equal to that
of ARB alone.
Effect of L-Carnitine and ARB on LPS Signaling
Hepatic LBP mRNA expression increased in the CDAA group
compared with that in the CSAA group (Figure 3B). In the ARB and
combination treatment groups, hepatic LBP mRNA expression was
significantly lower than that in the CDAA group (p < 0.05). Hepatic
LBP mRNA expression in the L-carnitine group was slightly lower,
but not significantly different.
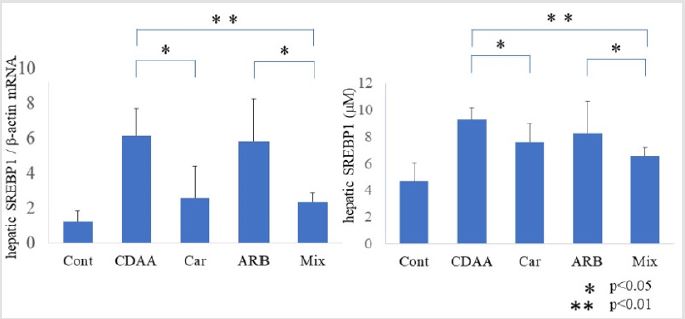
Inhibitory Effect of L-Carnitine and ARB on Oxidative Stress
Liver SREBP1 mRNA expression increased significantly over the
control after 8 weeks of CDAA feeding (p < 0.01). In the L-carnitine
group, SREBP1 mRNA expression was lower than that in the CDAA
group (p < 0.05; Figure 4A), but no significant effect was observed
in the ARB group. Under treatment with both L-carnitine and ARB,
SREBP1 mRNA expression was lower than that in the CDAA group,
but not significantly different from that with L-carnitine alone.
Similarly, the increase in hepatic TBARS level in mice fed the CDAA
diet, was decreased by L-carnitine treatment (p < 0.05; Figure 4B)
but did not change under ARB treatment. The effect of combination
of L-carnitine and ARB was similar to that of L-carnitine treatment.
These data suggest that the inhibitory effect of the combination
treatment on hepatic oxidative stress is associated with L-carnitine
rather than ARB.
Treatment with L-carnitine and ARB ameliorated liver fibrosis
and suppressed Ac-HSCs and oxidative stress in the rat NASH model.
Previously, we reported that a CDAA diet caused liver fibrosis
by increasing TNF-α [18]. In the present study, the combination
treatment with L-carnitine and ARB resulted in a synergistic antifibrotic
effect greater than that resulting from either drug alone.
L-Carnitine improved hepatic oxidative stress, whereas ARB
suppressed the activation of HSCs. NASH has various causative
factors, including inflammation and oxidative stress. Activation of
HSCs leads to liver inflammation and ultimately fibrosis, which is
governed by a complex network of autocrine/paracrine fibrogenic
signals promoted by the activation of HSCs and characterized by
the expression of α-SMA. The interaction between AT-II and Ac-
HSC plays an important role in liver fibrogenesis. We previously
reported that ARB directly inhibits Ac-HSC activation and that AT-II
is important for the upregulation of TLR4 expression via stimulation
of AT1R in Ac-HSCs [14]. Our results showed that ARB treatment of
rats with NASH reduces α-SMA-positivity and TNF-α, which leads to
an improvement in hepatic inflammation and fibrogenesis.
L-Carnitine is a vitamin-like dietary compound, synthesized
from the essential amino acids lysine and methionine [19]. The liver
is the main tissue for carnitine synthesis [6]. L-Carnitine is important
for the transport of long chain fatty acids into the mitochondrial
matrix via specialized acyltransferases [19]. Thus, L-carnitine
inhibits oxidative stress [20,21] and stimulates the β-oxidation
of fatty acids. Under L-carnitine deficiency, mitochondrial fatty
acid oxidation is impaired, and lipids accumulate in hepatocyte
cytoplasm, leading to the impairment of hepatic functions [22]. Our
results showed that L-carnitine treatment reduces hepatic SREBP
and TBARS in a rat model of NASH, leading to an improvement in
hepatic oxidative stress. In contrast, hepatic TNF-α, LBP, and α-SMA
were unchanged by L-carnitine treatment. Several studies have
indicated the effectiveness of L-carnitine for liver oxidative stress
and inflammation; however, its effectiveness for liver fibrosis is not
known [23,24].
In this study, we examined the inhibitory effect of the
combination of L-carnitine and ARB on liver fibrosis. As previously
reported, ARB improved only liver fibrosis. Furthermore, L-carnitine
slightly improved liver fibrosis without a significant difference.
However, the combination of L-carnitine and ARB resulted in
a significant improvement in liver fibrosis compared with that
of ARB alone. Overall, the combination of L-carnitine and ARB
showed a synergistic effect on liver fibrosis. This study was notably
limited using only one model animal. It is also difficult to detect
the signal transduction of L-carnitine for liver fibrogenesis using
in vitro study, as it does not directly affect hepatic parenchymal
cells. In conclusion, we found that simultaneous administration
of L-carnitine and ARB exerted a potent and synergistic inhibitory
effect on hepatic fibrosis compared with either agent alone by
suppressing oxidative stress and Ac-HSC proliferation, respectively.
This combination therapy could be useful for inhibiting NASH
progression in clinical applications.
We would like to thank Editage (www.editage.com) for English
language editing.
The authors declare no conflict of interest.
- Day CP, James OF (1998) Steatohepatitis: a tale of two “hits”? Gastroenterology 114(4): 842-845.
- Tilg H, Moschen AR (2010) Evolution of inflammation in nonalcoholic fatty liver disease: the multiple parallel hits hypothesis. Hepatology 52(5): 1836-1846.
- Musso G, Gambino R, Cassader M (2010) Gut microbiota as a regulator of energy homeostasis and ectopic fat deposition: mechanisms and implications for metabolic disorders. Curr Opin Lipidol 21(1): 76-83.
- Rotman Y, Koh C, Zmuda JM, Kleiner DE (2010) The association of genetic variability in patatin-like phospholipase domain-containing protein 3 (PNPLA3) with histological severity of nonalcoholic fatty liver disease. Hepatology 52(3): 894-903.
- Dongiovanni P, Petta S, Maglio C (2015) Transmembrane 6 superfamily member 2 gene variant disentangles nonalcoholic steatohepatitis from cardiovascular disease. Hepatology 61(2): 506-514.
- Ali SA, Faddah L, Abdel-Baky A, Bayoumi A (2010) Protective effect of L-carnitine and coenzyme Q10 on CCl4-induced liver injury in rats. Sci Pharm 78(4): 881-896.
- Rebouche CJ, Seim H (1998) Carnitine metabolism and its regulation in microorganisms and mammals. Annu Rev Nutr 18: 39-61.
- Longo N, Amat di San Filippo C, Pasquali M (2006) Disorders of carnitine transport and the carnitine cycle. Am J Med Genet C Semin Med Genet 142C(2): 77-85.
- Yoshiji H, Kuriyama S, Noguchi R, Fukui H (2004) Angiotensin-I converting enzyme inhibitors as potential anti-angiogenic agents for cancer therapy. Curr Cancer Drug Targets 4(7): 555-567.
- Yoshiji H, Yoshii J, Ikenaka Y, Noguchi R, Tsujinoue H, et al. (2002) Inhibition of renin-angiotensin system attenuates liver enzyme-altered preneoplastic lesions and fibrosis development in rats. J Hepatol 37(1): 22-30.
- Yoshiji H, Kuriyama S, Yoshii J, Ikenaka Y, Noguchi R, et al. (2001) Angiotensin-II type 1 receptor interaction is a major regulator for liver fibrosis development in rats. Hepatology 34: 745-750.
- Yoshiji H, Noguchi R, Ikenaka Y, Namisaki T, Kitade M, et al. (2009) Losartan, an angiotensin-II type 1 receptor blocker, attenuates the liver fibrosis development of non-alcoholic steatohepatitis in the rat. BMC Res Notes 2: 70.
- Yoshiji H, Noguchi R, Ikenaka Y, Kaji K, Aihara Y, et al. (2011) Cocktail therapy with a combination of interferon, ribavirin and angiotensin-II type 1 receptor blocker attenuates murine liver fibrosis development. Int J Mol Med 28(1): 81-88.
- Shirai Y, Yoshiji H, Noguchi R, Kaji K, Aihara Y et al. (2013) Cross talk between toll-like receptor-4 signaling and angiotensin-II in liver fibrosis development in the rat model of non-alcoholic steatohepatitis. J Gastroenterol Hepatol 28(4): 723-730.
- Kawaratani H, Tsujimoto T, Kitazawa T (2011) Therapeutic effects of cytokine modulator Y-40138 in the rat alcoholic liver disease model. J Gastroenterol Hepatol 26(4): 775-783.
- Noguchi R, Yoshiji H, Ikenaka Y, Kaji K, Aihara Y et al. (2013) Dual blockade of angiotensin-II and aldosterone suppresses the progression of a non-diabetic rat model of steatohepatitis. Hepatol Res 43(7): 765-774.
- Du Plessis J, Vanheel H, Janssen CE, Roos L, Slavik T, et al. (2013) Activated intestinal macrophages in patients with cirrhosis release NO and IL-6 that may disrupt intestinal barrier function. J Hepatol 58(6): 1125-1132.
- Kawaratani H, Tsujimoto T, Kitazawa T (2008) Innate immune reactivity of the liver in rats fed a choline-deficient L-amino-acid-defined diet. World J Gastroenterol 14(43): 6655-6661.
- Ozsoy SY, Ozsoy B, Ozyildiz Z, Aytekin I (2011) Protective effect of L-carnitine on experimental lead toxicity in rats: a clinical, histopathological and immunohistochemical study. Biotech Histochem 86(6): 436-443.
- Annadurai T, Vigneshwari S, Thirukumaran R, Thomas PA, Geraldine P (2011) Acetyl-L-carnitine prevents carbon tetrachloride-induced oxidative stress in various tissues of Wistar rats. J Physiol Biochem 67(4): 519-530.
- Shaker ME, Houssen ME, Abo-Hashem EM, Ibrahim TM (2009) Comparison of vitamin E, L-carnitine and melatonin in ameliorating carbon tetrachloride and diabetes induced hepatic oxidative stress. J Physiol Biochem 65(3): 225-33.
- Xia Y, Li Q, Zhong W, Dong J (2011) L-carnitine ameliorated fatty liver in high-calorie diet/STZ-induced type 2 diabetic mice by improving mitochondrial function. Diabetol Metab Syndr 15: 3-31.
- Ishikawa H, Takaki A, Tsuzaki R, Yasunaka T (2014) L-Carnitine Prevents Progression of Non-Alcoholic Steatohepatitis in a Mouse Model with Upregulation of Mitochondrial Pathway. PLoS One 9(7): e100627.
- Demiroren K, Dogan Y, Kocamaz H, Ozercan IH (2014) Protective effects of L-carnitine,N-acetylcysteine and genistein in an experimental model of liver fibrosis. Clin Res Hepatol Gastroenterol 38(1): 63-72.

 Research Article
Research Article