Abstract
Sperm cryopreservation is a process whereby sperm are generally stored frozen at -196°C in a tank filled with liquid nitrogen (LN2) and a standard practice used in infertility treatment. However, the LN2 freezing method can be regarded as a fragile preservation approach. Development of an alternative storage method that can permit storage at room temperature and does not require periodic replenishment of a freezing agent is necessary. An alternate storage method that is attracting attention is the lyophilization (freeze-drying) method, although the lyophilization method has not yet been clinically applied to humans. We introduce a new freeze-drying method (micropowder- dry) that may control the formation of ice crystals in cells.
Keywords: Sperm Preservation; Lyophilization; Micro-Powder-Dry
Introduction
Sperm cryopreservation is a process whereby sperm are
generally stored frozen at -196°C in a tank filled with liquid
nitrogen (LN2) after freezing by the LN2 vapor freezing method. It
is a standard practice used in infertility treatment, including shortterm
storage for artificial insemination and in vitro fertilization,
as well as long-term storage before cancer treatment for fertility
preservation. In particular, young patients may require long-term
storage up to future use thus, there is a need for a stable sperm
cryopreservation method.
However, liquid nitrogen is highly volatile; therefore, even if
sperm is stored in a special tank, regular replenishment of LN2 is
essential. Unfortunately, there have been cases where irreversible
loss of stored samples occurred due to insufficient supplementation
of LN2 attributed to natural disasters or human errors, and this has
become a major social problem. For example, in 2018, a University
Hospital in U.S.A. suffered an accident in which about 4000 eggs
and embryos were lost following rising temperature caused by
failure of the freezer storage and lack of replenishment with LN2
[1]. This example is not a unique case, and similar cases have
sporadically occurred in other countries. Such problem arises
because regular replenishment of LN2 is indispensable in the LN2
freezing method, which can be regarded as a fragile preservation
approach. Therefore, development of an alternative storage method
that can permit storage at room temperature and does not require
periodic replenishment of a freezing agent is necessary.
Sperm Preservation Methods
Extracellular Freezing and Intracellular Freezing
Depending on the rate of freezing, cells freeze via two different ways. When the freezing rate is slow, the water around the cells is first frozen, and the water in the cells move out of the cells to become ice, and then rapidly solidifies at a temperature at which the concentration of solutes in the cells increases. This process is called extracellular freezing, whereby the cell membrane is distorted due to cell volume reduction and the extracellular membrane breaks down the cell membrane [2]. The cell is damaged because it loses semi-permeability [3]. In contrast, when the freezing rate is high, the water is frozen inside the cell due to insufficient time for water movement to the outside of the cell. This is called intracellular freezing, and during this process, the cell tissue is destroyed and damaged when coarse ice crystals grow inside the cell [2]. However, cell damage is unlikely to occur if fine ice crystals are generated inside the cell through ultra-rapid cooling [4].
Lyophilization (Freeze-Drying)
An alternate storage method that is attracting attention is
the lyophilization (freeze-drying) method used for long-term
storage of foods and pharmaceuticals. In the conventional drying
method, liquid water is vaporized into water vapor. In contrast,
the lyophilization method, in which ice directly sublimates into
vapor without any intermediate liquid phase through freezing
followed by depressurizing with a vacuum pump, enables steady
maintenance of the concentration of the solution. Thus, cell tissue
changes are less likely to occur using this method. However, coarse
ice crystals will grow during the longer drying time that follows the
cell freezing stage, and cells can become damaged. In addition, if
the drying causes excessive dehydration, this may lead to loss of
the bound water that helps maintain the tissue structure in the
cell, and the cell becomes damaged [3]. Therefore, in order to use
the freeze-dry method without damaging the cells, it is necessary
to sublimate before ice crystal growth by rapid drying, although
this drying should not go beyond the extent where cells become
excessively dehydrated. The lyophilization method allows storage
of sperm at 4°C or room temperature for a long time, does not
require regular replenishment of a freezing agent, and is relatively
easy to manage. Mouse spermatozoa after freeze-drying can be
stored at room temperature for more than one year [5], and can
withstand rapid temperature change [6]. In addition, sperm
processed by the lyophilization method can withstand storage
in space, which is a high radiation environment [7]. These are
clearly advantageous in terms of storage stability and convenience
compared to conventional methods currently used in reproductive
medicine. Although studies using lyophilization methods have been
conducted in various animal species including humans, sperm
motility after rehydration has not been recovered in all animal
species thus far. This is partly because the sperm cell membrane is
destroyed [8].
The main causes of the destruction of the cell membrane are
likely the ice crystal formation in the solution that occurs during
freezing and the pressure and temperature during drying. The
current approach to lyophilization of sperm mainly involves the
collection of the motile sperm in a lyophilized solution, then freezing
in LN2, followed by evaporating the water by vacuum drying in a dry
layer [9]. In mice and rabbits, it has been confirmed that fertilizing
ability is maintained with freeze-dried sperm [10,11], whereas,
there has been no successful application in humans. If motility of
sperm is not restored, then pregnancy using artificial insemination
or in vitro fertilization, which requires many motile sperm, cannot
be achieved. In mice and rabbits, the functions necessary for
fertilization and embryo development are maintained even in dead
sperm, and intracytoplasmic sperm injection (ICSI) has enabled
the use of spermatozoa with disrupted cell membranes. However,
in humans, the fertilization rate of ICSI using motionless sperm
is extremely low; therefore, the freeze-drying method has not yet
been clinically applied to humans. Acquisition of motile sperm
after freeze-drying is expected to contribute to the successful
reproduction in male cancer patients after long-term treatment, as
well as the preservation of rare species. For successful preservation
of human sperm, it is therefore necessary to select a method that
prevents damage to the sperm cell membrane and suppresses the
formation of ice crystals of moisture inside and outside the cell.
Here, we introduce a new freeze-drying method that may control
the formation of ice crystals in cells.
Micro-Powder-Dry (μPD) Process and Application for Cell Freeze-Drying
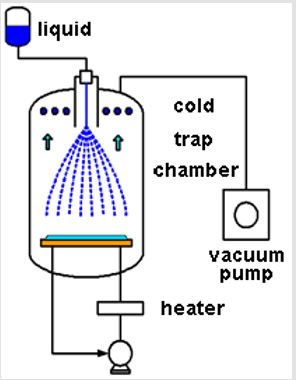
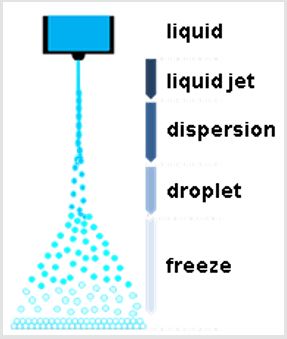
Micro-Powder-Dry (μPD) is a unique freeze-drying process (ULVAC, Inc, Japan Patent P3942093). In the μPD process, some liquid containing some ingredients is injected into a vacuum chamber through a nozzle (Figure 1). The liquid jet is dispersed due to its instability and the droplets are frozen rapidly by the evaporation heat (Figure 2). Then, the frozen particles are dried by sublimation in the vacuum chamber and freeze-dried powder can be obtained in the spherical shape (Figure 3). Furthermore, an additional process for rapid drying has been developed, in which the sublimation is accelerated by radiation heat (ULVAC, Inc, patent pending).
For freezing cells without damaging cell structures, large intracellular ice crystals should be prevented [12]. Using the μPD process, small ice crystals can be formed due to rapid freezing. In the thermodynamics calculation of a 100μm diameter droplet of pure water, it takes 6 msec for its temperature to fall from 20 to -35°C in a vacuum. Even though small ice crystals are formed, cell structures may be damaged by the grain growth. The rapid drying process using radiation heat could make ice sublimate before the grain growth of ice crystals. In another aspect, aggregation of cells should be prevented for cell freeze-drying. Using the μPD process, the distribution of cells is fixed in micro-droplets when frozen, then ice is sublimated without cell aggregation. For these reasons, μPD is expected to freeze-dry cells with minimal damage. Based on a preliminary experiment, it was found that some freezedried collagenases retained more activity using the μPD process compared to the conventional freeze-dry process. This suggests that μPD could be a better approach for freeze-drying cells and could potentially be applied to the freeze-dried storage of human cells, especially sperm, and this will be examined in future studies.
Conclusion
The development of an alternative storage method that can permit storage at room temperature and does not require periodic replenishment of a freezing agent is necessary. The lyophilization method will be an alternate storage method, although it has not yet been clinically applied to human sperm, and needs to be improved in order to prevents damage to the sperm cell membrane and suppresses the formation of ice crystals of moisture inside and outside the cell. The μPD could be a better approach for freezedrying cells and could potentially be applied to the freeze-dried storage of human sperm, and this will be examined in future studies.
References
- (2019) Washington Post: Embryo storage bill seeks oversight of fertility centers and penalties for those that violate safeguards.
- Hiroshi Souzu (1980) KAGAKU TO SEIBUTSU 18: 78-87.
- Hiroshi Souzu (1991) Japanese journal of freezing and drying 37: 1-6.
- Eizo Asahina (1980) journal of freezing and drying 26: 46-51.
- Kamada Y, Wakayama S, Shibasaki I, Ito D, Kamimura S, et al. (2018) Assessing the tolerance to room temperature and viability of freeze-dried mice spermatozoa over long-term storage at room temperature under vacuum. Sci Rep 8(1): 10602
- Wakayama S, Ito D, Kamada Y, Yonemura S, Ooga M et al. (2019) Tolerance of the freeze-dried mouse sperm nucleus to temperatures ranging from -196°C to 150°C. Sci Rep 9(1): 5719
- Wakayama S, Kamada Y, Yamanaka K, Kohda T, Suzuki H et al. (2017) Healthy offspring from freeze-dried mouse spermatozoa held on the International Space Station for 9 months. PNAS 114(2): 5988-5993.
- Kusakabe H, Szczygiel MA, Whittingham DG, Yanagimachi R (2001) Maintenance of genetic integrity in frozen and freeze-dried mouse spermatozoa. PNAS 98(24): 13501-13506.
- Kaneko T (2015) Simple sperm preservation by freeze-drying for conserving animal strains. Methods Mol Biol 1239: 317-329.
- Wakayama T, Yanagimachi R (1998) Development of normal mice from oocytes injected with freeze-dried spermatozoa. Nat Biotechnol 16(7): 639-641.
- Liu JL, Kusakabe H, Chang CC, Suzuki H, Schmidt DW et al. (2004) Freeze-dried sperm fertilization leads to full-term development in rabbits. Biol Reprod 70(6): 1776-81.
- Shimada K, Asahina E (1975) Visualization of intracellular ice crystals formed in very rapidly frozen cells at -27degree C. Cryobiology 12(3): 209-218.

 Short Communication
Short Communication