Research Article
Single Dose Toxicity Study of Polysaccharide VI
Conjugate Vaccine in Sprague Dawley Rats
Reynaldo Oliva Hernández1*, Mildrey Fariñas Medina1, Juan Francisco Infante1, Tamara Hernández
Salazar1, Yohani Pérez Guerra2, Ambar Oyarzabal2 and Sonsire Fernández Castillo1
Author Affiliations
1Finlay Institute of Vaccine, Havana, Cuba
2National Center of Scientifics Research, Natural Products Center, Cuba
Received: November 28, 2019 | Published: December 05, 2019
Corresponding author: Reynaldo Oliva Hernández, Finlay Institute of Vaccine, Havana, Cuba
DOI: 10.26717/BJSTR.2019.23.003934
Typhoid fever continues to be a major public health problem according with estimates
of World Health Organization. Conjugation of polysaccharides to an immunogenic
protein revert the Thymus independent pattern of polysaccharides to a T-dependent
pattern and induce immune response in infants. The aim of this work was to evaluate
the toxicological profile a conjugate candidate vaccine against this disease through
single dose study in Sprague Dawley rats. Animals were observed daily for local and
systemic toxicity symptoms. Also, body weight, water and food consumption, immune
response, temperature and local inflammation in the site of injection were evaluated.
Gross necropsy was made at the end of the study to each rat, selected organs were
weighed, and a full range of tissues was preserved for histological studies. The vaccine
not evidenced clinical symptoms, deaths, local effects or adverse systemic toxicological
change. Therefore, this vaccine may be considered potentially non-toxic for human.
Summary: Typhoid fever remains a major public health problem according to
estimates by the World Health Organization. The conjugation of polysaccharides to an
immunogenic protein reverses the thymus pattern independent of polysaccharides to a
dependent T pattern and induces an immune response in children. The objective of this
work was to evaluate the toxicological profile of a conjugate vaccine candidate against
this disease, using a single dose trial in Sprague Dawley rats. Animals were observed
daily to detect symptoms of local and systemic toxicity. In addition, body weight,
food and water consumption, immune response, temperature and local inflammation
at the injection site were evaluated. At the conclusion of the study, macroscopic
anatomopathology was performed on all animals, selected organs were weighed and
all organs processed for histological studies. The vaccine showed no clinical symptoms,
deaths, local effects or adverse systemic toxicological changes. Therefore, this vaccine
can be considered potentially non-toxic to humans.
Keywords: Conjugate Vaccine; Salmonella
Typhi; Single Dose; Toxicity
Salmonella typhi is a Gram-negative bacterium that produce
a systemic infection known as typhoid fever. Salmonella enterica
serovar Typhi (. Typhi) continues to be a significant cause of
morbidity and mortality in endemic regions. This disease produce
annually around of 26 million people are culture positive for S.
Typhi with an associated 216,000 – 600,000 deaths annually,
principally affecting school children (5-15 years) and younger [1].
The continued burden of typhoid fever and the alarming spread
of antibiotic resistant strains lead vaccination as an important
control measure. Two typhoid vaccines are widely available, Ty21a
(oral) and Vi polysaccharide (parenteral). However, these vaccines
are not recommended for use children fewer than 2 years of age
because are not efficacy, the group with the highest mortality rate
(4% vs 0.4%) compared to the 5-15 years old group [2]. Both
typhoid vaccines have other disadvantages; the attenuated strain
component of oral Ty21a vaccine requires multiple doses (3 or 4) to
induce an effective immune response, which is complex to perform
in many developing countries [3]. Moreover, vaccines based on non- conjugated polysaccharides do not induce immunological memory
therefore revaccinations is necessary every certain period, and
in addition are not effective in infants [4]. It´s well known that
conjugation of polysaccharides to carrier proteins produces a
reversal in the pattern of response induced thymus independent
polysaccharide to a thymus-dependent pattern, which causes the
induction of immune response and long-term effective in infants
[5]. Newer typhoid conjugate vaccines are at varying stages of
development and use. In similar way, Finlay Institute of Vaccine
is developing of a new conjugate Vi polysaccharide vaccine for
increase his products profile and for contribute in help to prevent
typhoid fever. The objective of this study was to perform the
preclinical safety testing through the acute toxicity by single dose
as part of mandatory aspects before clinical essays.
Animals and Husbandry
Female and male SD rats were purchased from CENPALAB,
Havana Cuba (from Spanish: Centro Nacional para la Producción de
Animales de Laboratorio) at an age of 7-8 weeks and were housed in
Tecniplast® rat cages at the Animal Care Facility at Finlay Institute
of Vaccine. Dimensions and model: 1354G Eurostandard Type IV,
595 x 380 x 200 mm, floor area 1820 cm, PEI plastic and BPA-Free.
Rats were provided specialized feed for rodents (ALYco®) and the
water used was provided in acidified (2.5-2.7 pH) water bottles
(750 mL volume), both were available ad libitum. The animal room
was maintained at a temperature of 22 ± 2°C and a relative humidity
of 55 ± 5%. These parameters were recorded daily in addition to
maintaining 12-hour light and dark cycles. Rats were allowed to
acclimatize to their surroundings for one week prior to the start of
the experimental protocol and were randomly placed into groups of
10. All protocols were approved by the Animal Care Committee and
the Biosafety Committee at Finlay Institute of Vaccine.
Vaccine and Experimental Design
Each vaccine vial contained 10 μg of Capsular polysaccharide
Vi of Salmonella Typhi per dose (in 0.5 mL, human dose) conjugated
to Diphtheria toxoid as active pharmaceutical ingredient and
auxiliary substances used as placebo, as show next. The study was
designed according to the World Health Organization guidelines
on nonclinical evaluation of vaccines and typhoid conjugate vaccines
[6,7]. Ten animals were randomly assigned to each group of
treatment with the help of a list generated by the Aleator software
application (IFV, Habana, Cuba; version 1.2, 1999). The dose was
administered intramuscularly, and animals received 4 μg of vaccine
dose in a volume of 0.2 mL (40 % of human dose), divided in two
sites (both legs). The latter corresponds to the maximum allowable
volume according to the chosen administration route and host species
[8], which constitutes the reference to establish the upper limit
for dose and toxicity in the proposed experimental design. Control
animals received phosphate buffer solution (PBS) administered in
identical conditions than placebo and vaccinated, who representing
the three groups in study. The assay lasted two weeks (14 days).
Clinical Examination, Body Weight, Water and Food
Consumption
The animals underwent two time at day clinical examinations
during first 72 h and then daily with the objective of detecting any
behavioral variation or sign of toxicity such as changes in skin and
fur, in eyes and mucous membranes or somatomotor activity. At the
time of inoculation, rats were weighed to determine their starting
weight at the beginning of the study. All rats were identified by the
ear punch-out method and weighed at weekly intervals to monitor
their weight as a measure of toxicity. Water and food consumption
were measured at the start of the experimental design and on
alternate days thereafter; daily water and food consumption (mL or
g/animal/day respectively) were calculated based on the amount of
food and water consumed over the span of a week.
Body Temperature and Muscle Diameter
The body temperature of the animals was measured rectally
using a clinical thermometer thin of mercury (Hemeco®, China).
This procedure had a duration of one minute. Muscle volume
was measured using a caliper (Scala®, Germany), measuring the
diameter of the limb (both legs) before inoculation, both operations
were performed at the times 0, 8, 24, 48 and 72 hours post
inoculation. These operations were carried out in a similar way to
those described above [9].
Hematological and Immunological Studies
A terminal blood sample was taken from Isoflurane - anesthetized
rats via cardiac puncture after 6 hours of fasting immediately
prior to euthanasia (on day 14). The collected blood was divided in
two samples using Eppendorf Tubes® (1.5 mL). One sample was
treated with EDTA as Anticoagulant for hematological evaluation.
Another sample was allowed to clot at 4 °C for 30 minutes, and centrifuged
at 7000rpm for 10 minutes, and serum was decanted and
frozen at -70 °C for immunological test. The following hematological
parameters were determined: Red blood cell count, hemoglobin
concentration, hematocrit, platelets, leukocytes, lymphocytes,
monocytes, mean corpuscular volume, mean corpuscular hemoglobin,
and mean corpuscular hemoglobin concentration. A slide
smear was made from a single drop of whole blood. Hematological
data was generated from an Auto Hematology Analyzer (Model
Mindray® BC-2800 Vet., China). Sera collected from rats of 0 and 14
day were analyzed for IgG anti polysaccharide Vi response.
Briefly, ELISA plates were coated 100 μL of 3μg/mL solution
of Poly-L-Lysine (Sigma®, EUA) and incubated for 1 h at room
temperature. After 4 washes with PBS, the plates were coated
overnight (4°C) with 100 μL of Vi polysaccharide at 5 μg/mL in
PBS. The plates were blocked with 1% (w/v) skim milk in PBS for
1 h at 37°C. After washing with PBS containing 0.05% Tween 20, duplicate serum samples (diluted 1:100 in 3% skin milk PBS-T)
were added (100 μL/well) and incubated for 1 h at 37°C. Then, the
plates were incubated with 100 μL/well of alkaline phosphatasegoat
anti-rat IgG conjugate (Sigma®, EUA) at a dilution of 1:8000
in blocking solution. The reaction was revealed with p-nitrophenol
phosphate tablets (Sigma®, EUA) dissolved in 0.1 M sodium citrate
buffer, pH 5. Fifteen minutes later, the reaction was stopped with a
2 N NaOH solution. Absorbance at 405 nm was read using a Titertek
Multiskan (Flow Laboratories) reader. ELISA data was processed
with Program ELISA for Windows version 2.15 (Centers for Disease
Control and Prevention). Anti-Vi IgG levels were expressed in
ELISA Units using a hyperimmune sera as standard curve with an
assigned value of 100 ELISA Units. The seroconversion rate was
also calculated. It was defined as a 4-fold increase in the IgG levels
from 0 to 14 day.
Anatomopathological Studies and Organ Weights
The anatomopathological studies for gross necropsy were
performed immediately after euthanasia examining all organs and
the sites of vaccination. Representative tissues for histopathological
were collected and immersion fixed in 4 % Neutral Buffered
Formalin and process by paraffin inclusion and Haematoxylin -
Eosin stain (HE). The curt and width of tissues (3 to 4 microns)
were made following WHO guidelines and similar to other studies
[6,9,10]. Solid or parenchymal organs (heart, lungs, spleen, liver and
kidneys) and thymus were removed, and weights were recorded.
They are expressed as relative weight and were calculated by the
following equation: RW = (OW x 100)/EEW, that’s means, relative
weight (RW) is equal to organ weight (OW) per 100 divided by
euthanasia end weight.
Statistical Analysis
Statistical analyses were performed using Graph Pad Prism 5.
Multiwise group analyses were performed using a nonparametric
ANOVA with a Dunn’s post-hoc test. The significance level was
adjusted for multiple comparisons using Bonferroni test. Data were
considered significant when p ≤ 0.05.
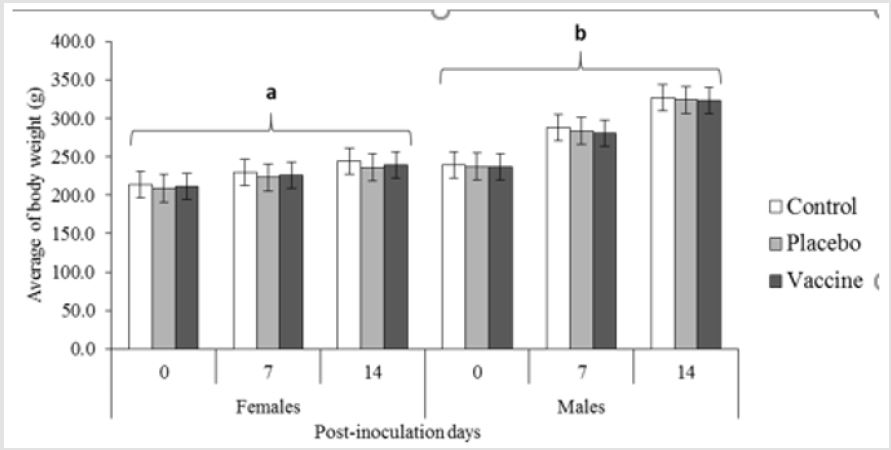
No mortality or abnormal clinical signs were noted during the
study. All of the rats increased their body weight during the 14 days
of the study (Figure 1). The weight increase curves of the rats are
similar to those observed for this species and in line with the growth
curves available from Charles River and the reported in our animals
facility [9,10] no statistical differences was observed between
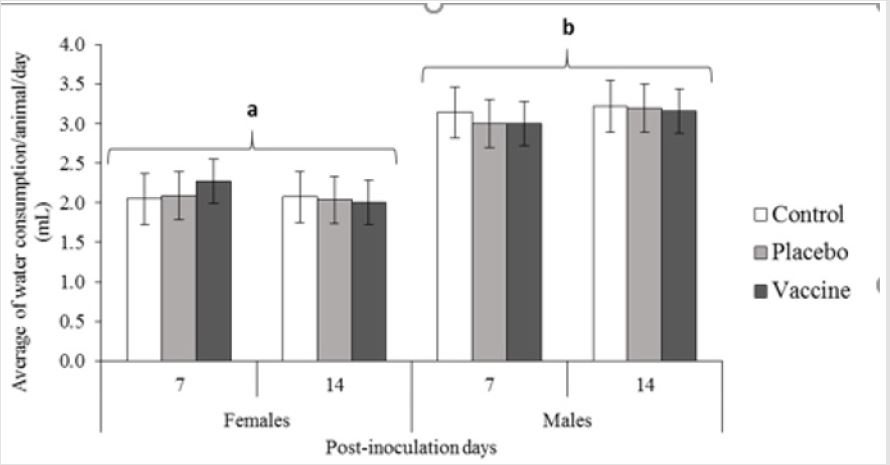
vaccine and control groups. We also evaluated the amount of water
and food intake (Figures 2 & 3) by the rats over the span of the
experimental design, which was not significantly different between
any of the groups evaluated in the study for water consumption. (p
≥0.05). However, as usually the males drank more than females on
average being 3.45 mL/day vs. 2.43 mL/day showing significantly
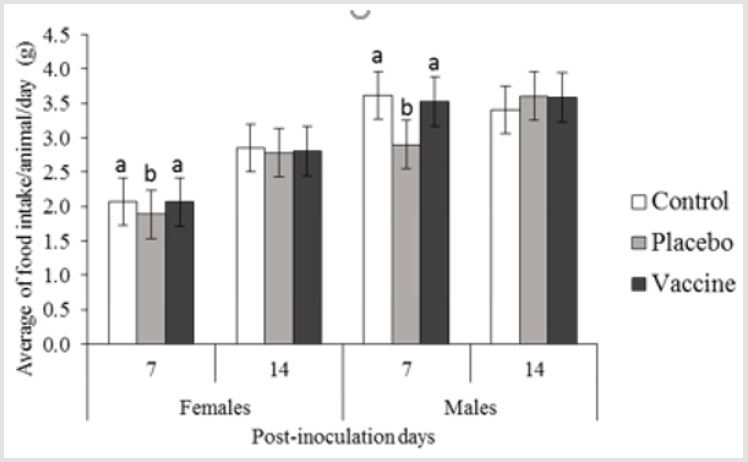
different between sexes (p ≤0.05). Regarding to food intake, were
founded differences between treatment groups for both sexes, the
placebo groups, differ from vaccinates and controls (p ≤0.05) during
the first 7 days. Nevertheless, the ranges of individual consumption
for all groups were between those reported for the species by age
and weight [9-11]; males consumed more than females on average,
being 3.53 g/day for males and females 2.43 g/day. In general, these
results were consistent with those observed historical values for
rats of this category in our facilities [9-11].
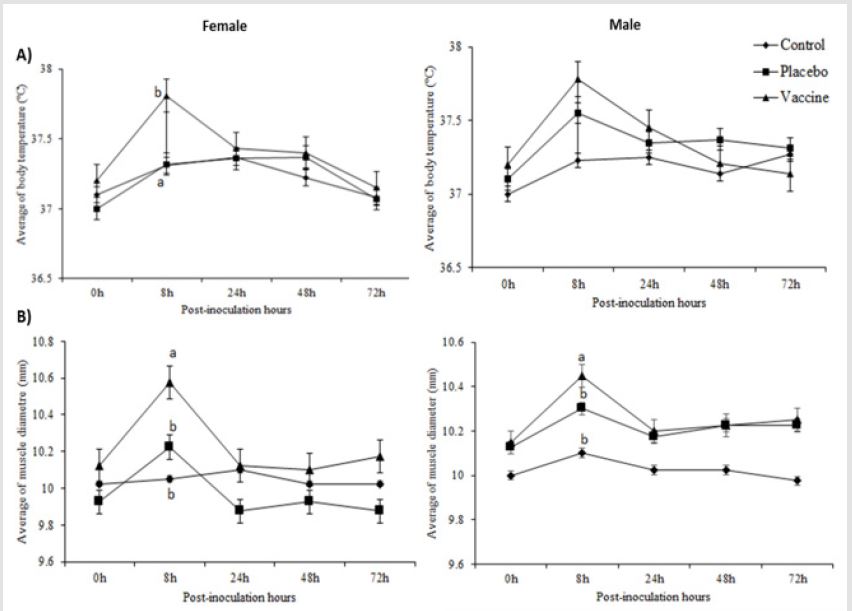
Rats don’t evidence fever during the study (Figures 4 A), her
behavior was in physiological range to the species (CCAC, 2010).
However, there was a significant increase in temperature during
the first 8 to 24 hours in the female rats from vaccine group as
averaging 37,8 ºC (three female with 38.2 ºC) with respect to control
and placebo groups. Although, a similar average was observed for
males rats at 8 hours post-vaccine inoculation without difference
between groups (p≥0.05). In order to assess the inflammation
induced by the vaccine administered intramuscularly, the muscle
diameter of the legs was measured before and after receiving the
treatments. We saw differences between vaccinated animals with
respect to placebos and controls groups for both sexes at 8 hours
post-inoculation (p≤0.05) (Figures 4B).
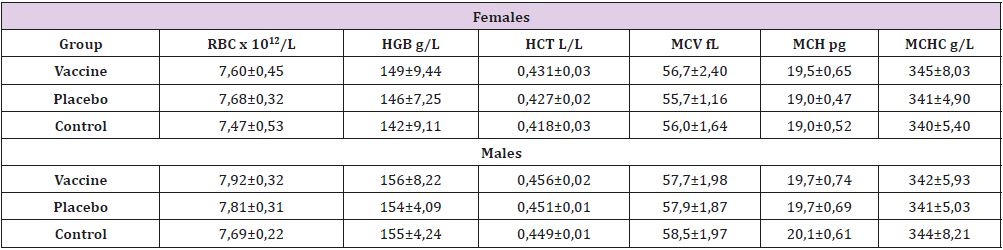
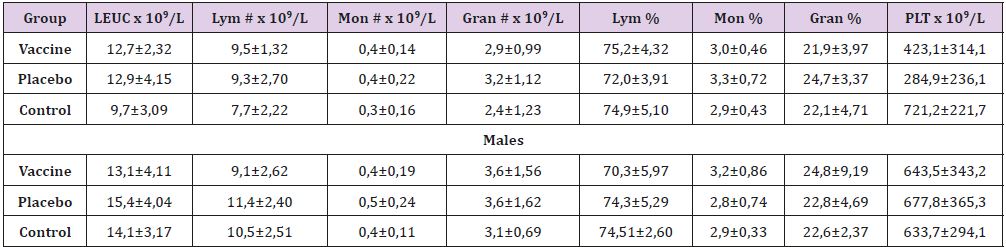
In general, the blood parameters assessed was within the
physiological ranges described to the species (Table 1) with
similarity between sexes and treatment groups. In the series of
cells red, although the concentration of hemoglobin in the three
groups was slightly higher than that reported in the literature
reference [12-14] this parameter didn’t differ between them and
happened similar with white blood cell (Table 2), where we don´t
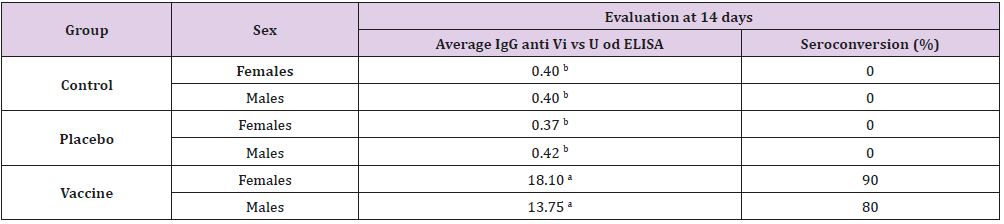
found differences. Immunological response of the rats in our
study was similar to previously reported [11]. To both sexes in the
vaccine group, the antibody average response (18.01 to female and
13.75 to male) at 14 days was significantly higher (p≤0.05) than
the placebo and control groups (0.49 average, Table 3) reinforcing
the relevance of the selected animal model and a good immune
response to the conjugated polysaccharide Vi vaccine. On the other
hand, seroconversion was higher in vaccinated females (90%)
than in males (80%). Sex-based differences in immunity have
been previously described [11]. The immune factors that regulate
the complex immunoendocrine net, and the gender might have
a significant function in shaping the immune response and can
explain the disparities.
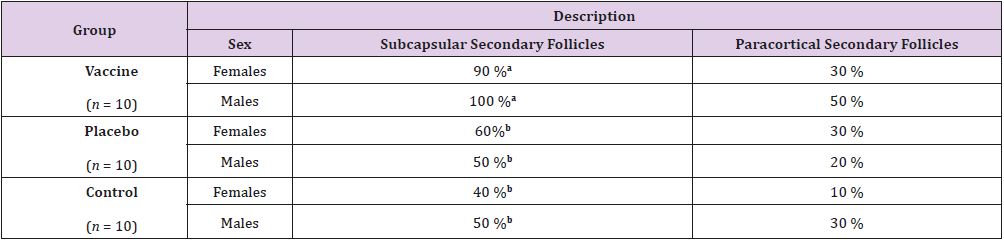
The gross necropsy studies performed on all organs and systems
for each of the rats studied didn’t show any lesions suggesting acute
toxicity. Administration sites showed no perceptible local changes.
While, some discrete processes of reaction from the immune system
at the level of regional lymph nodes near the site of inoculation
were observe as histological findings part (Table 4). Such as, the
presence of subcapsular secondary follicles in lymph nodes was
significant among male rats from vaccinated group respect to
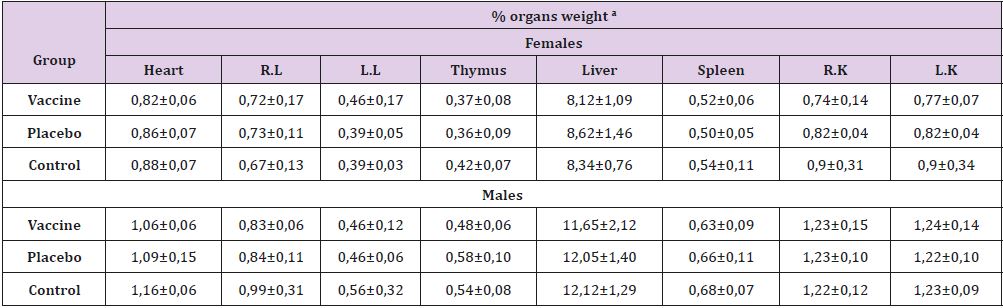
controls (p≤0.05). Regarding the relative weight of organs (Table
5), significant differences were observed between heart weight
of female’s rats and thymus of male’s rats, these was between the
vaccinated and placebo groups, but not with the control group (p
≤0.05). A joint analysis of the relative weights of the organs, as well
as the body weights of the animals under study, led us to conclude
that these variables were not affected by the applied treatment.
Today the experience with several polysaccharide–protein
conjugate vaccines (eg. meningococcal, pneumococcal vaccines
and others) has evidenced that conjugation can overcomes
many of the limitations associated with unconjugated bacterial
polysaccharides. On the ideas of this experience and to try to
address the limitations of the various typhoid vaccines described
above, several Vi polysaccharide–protein conjugate vaccines have
been developed. One of they could be our candidate vaccine in
preclinical development phase constituted by polysaccharides Vi
and conjugated to diphtheria toxoid. In a previously pilot essay the
conjugate polysaccharide Vi vaccine has shown immunogenicity
response and potential safety Fariñas et al., [11] and protective
effect in mice (data not shown). This study closes the previous
results, based on the absence of symptoms and mortality, where
the weight gain of the animals are correspondence with the
growth curves for species (Charles River, 2019). The body weight
is commonly considered in a wide range of toxicological studies
as a sensitive and general indicator of the toxicity of xenobiotic
[9,10,11,13,14]. This parameter have relation with water and food
consumptions, as well as health in general.
A relevant indicator for clinical trials is the reactogenicity of
the vaccines and the local response [15]. In this sense, temperature
and muscle diameter points out that the vaccine has a low
reactogenicity, because the local and systemic effects do not exceed
8 hours. The hematological studies reveal part of the internal
and systemic functioning, showing no physiological changes in
any of the animals [16]. These results are related to the absence
of macroscopic and microscopic pathological damage, as well as
the weight of the organs. On the other hand, the main histological
findings were in the lymphoid organs (popliteal and deep inguinal
lymph nodes). Effect directly related to the immunological response
to the vaccine in the animals that received it; is a local response
and similar to those reported in other vaccines [9,10,13]. This last
element reinforces the antibody titers detected by the ELISA in the
vaccinated rats, also supports that the conjugate polysaccharide
Vi vaccine is immunogenic in this biomodel. Together, the data
suggests that conjugate polysaccharide Vi vaccine is potentially
non-toxic to humans [17].
Conceptualization, RO, MF, JFI, SFL; Methodology, MF, RO, JFI;
Validation, RO, MF, TH; Formal Analysis, RO, JFI; Investigation, RO,
MF, JFI, TH, YP, AO, SF; Resources, SF, YP; Data Curation, RO, MF, SF,
Writing-Original Draft, RO, MF, TH, SF; Writing-Review & Editing,
RO, MF, JFI, TH, YP, AO; Visualization, RO, MF, JFI; Supervision, TH;
Project Administration, RO; Funding Acquisition, SF.
This work was supported by Salmonella typhi project from the
Finlay Institute of Vaccine, Havana, Cuba (IFV).
The authors declare no conflict of interest.
The authors are also thankful for the technical assistance by
Maria Onelia Gonzalez Socarras, Alex Quintero Perez, Darcy Nuñez,
Yolanda Valdes and to all people from animal facility.
- Crump JA, Luby SP, Mintz ED (2004) The global burden of typhoid fever. Bull WHO 82(5): 346-353.
- Geoffrey C Buckle, Christa L Fischer Walker, Robert E Black (2012) Typhoid fever and paratyphoid fever: Systematic review to estimate global morbidity and mortality for 2010. J Glob Health 2(1).
- (2010) Feedback from the regions and countries on the implementation of SAGE recommendations on typhoid vaccines.
- Jones C (2005) Vaccines based on the cell surface carbohydrates of pathogenic bacteria. An Acad Bras Cienc 77(2): 293-324.
- Stein KE (1992) Thymus-independent and thymus-dependent responses to polysaccharide antigens. J Infect Dis 165(Suppl 1): S49-S52.
- (2015) WHO Guidelines on nonclinical evaluation of vaccine. World Health Organization 927.
- (2013) World Health Organization. WHO, Guidelines on the quality, safety and efficacy of typhoid conjugate vaccines?
- Diehl KH, Hull R, Morton D, Pfister R, Rabemampianina Y, et al. (2001) A good practice guide to the administration of substances and removal of blood, including routes and volumes. J Appl Toxicol 21(1): 15-23.
- Oliva Hernández R, Fariñas Medina M, Infante Bourzac JF, Hernández Salazar T, Núñez Martínez D et al. (2019) Local tolerance study of the VA-MENGOC-BC® antimeningococcal vaccine in Sprague Dawley rats. Evaluation at 24 and 36 months of shelf. Vacci Monitor 28(1): 9-18.
- López Y, Pastor M, Infante JF, Díaz D, Oliva R, et al. (2014) Repeated dose toxicity study of Vibrio cholerae-loaded gastro-resistant microparticles. J Microencapsul 31(1): 86-92.
- Mildrey Fariñas Medina, Reynaldo Oliva Hernández, Juan F Infante Bourzac, Yolanda Valdez Abreu, Darcy Nuñez Martínez, et al. (2014) Pilot trial of immunogenicity and preclinical toxicity of the Salmonella typhi conjugate vaccine in Sprague Dawley rats. Sertox.
- Avelina Caridad León Goñi, Diuris Blanco, Amelia Peña, Marisel Ronda, Bárbara O González, et al. (2011) Hematological and biochemical values of Sprague Dawley rats produced in CENPALAB, Cenp: SPRD. Electronic Veterinary Magazine 22(11).
- Gutiérrez A, Gámez R, Noa M, Mas R, Arencibia D, et al. (2011) One year oral Toxicity of D-004, a lipid extract from Roystonea regia fruits, in Sprague Dawley rats. Food and Chemical Toxicology 49(2011) 2855-2861.
- Tamargo B, Bungao S, Fleitas C, Marquez Y, Infante JF, et al. (2019) Immuno-toxicological Evaluation of the Adjuvant Formulations for Experimental Anti-meningococcal Vaccines without Aluminum Hydroxide. ResearchGate 70(4): 1251-1275.
- Ochoa RF, Baró IM, Menéndez J, Triana T, Mirabal M, et al. (2006) Reactogenicidad e inmunogenicidad de una nueva vacuna de toxoide tetánico y diftérico con concentración reducida en adolescentes cubanos. VacciMonitor15(2): 13-17.
- CD® (Sprague Dawley) IGS Rat Details.
- (2010) Guide for the care and use of laboratory animals. (8th)., 2010. The National Academies Press.

 Research Article
Research Article