Abstract
Introduction: Glioblastoma (GBM) is the most aggressive primary brain cancer. A number of stem cell markers, including cancer and specifically GBM, have been identified and well described in literature. However, no attempts have been made to determine a relation between the expression of cancer stem cell markers in GBM and morphological features of this tumor. Therefore, the present study attempts to compare the expression of stem cell markers found in GBM tissue with selected features of tumor malignancy.
Material and Methods: 21 GBM samples were tested. In the assessment of GBM histological features, the following were considered: nuclear pleomorphism, Ki-67 labeling index and endothelial proliferation. The expression of CD133, GFAP, Nestin, βIIItubulin, Oct-4, Nanog, Olig2 and Rex was analyzed by reverse transcription polymerase chain reaction.
Results: A significantly higher expression of CD133 (p = 0,0007) and Nestin (p = 0,0216) were found in GBM with evident nuclear pleomorphism. We found also significantly higher expression of CD133, Nestin and Olig2 in patients with a Ki-67 labeling index over 15% (p = 0.0033, p = 0.0101, and p = 0.0066).
Conclusion: The expression of biomarkers of stem cells in GBM is associated with a greater severity of anaplasia and tumor proliferative activity. So, it can be assumed that the aggressiveness of GBM is at least partly due to the presence of cancer stem cells in the tumor mass. Due to the resistance of these cells to radio- and chemotherapy, the described phenomena may be associated with lower therapeutic efficacy in patients with GBM.
Keywords: Glioblastoma Multiforme; Cancer stem cells; Markers; Tumor
Abbreviations: GBM: Glioblastoma Multiforme; qRT-PCR: Quantitative Reverse Transcriptase Real-Time PCR; GFAP: Glial Fibrillary Acidic Protein
Introduction
In the current classification of the World Health Organization,
gliomas are classified into four grades (G1-4). The most malignant
in this group of CNS tumors is Glioblastoma multiforme (GBM).
Despite advances in neurosurgery, radiation and chemotherapy,
the average survival rate is only several months [1]. GBM is a
cancer with a very diverse morphological image with features of
pronounced anaplasia.
The previous decade has been a period of interest in cancer
stem cells, identified in a variety of primary tumors. Stem cells
in GBM may be isolated from other cells in the primary tumor of
the brain with the use of markers of neural stem cells, i.e. CD133,
Nestin, βIII-tubulin, as well as markers of glial cells, for example
GFAP. Currently, it is being suggested that transcription factors
such as Nanog, Oct-4, Rex and Olig2 can be used as biomarkers
of cancer stem cells and help identify these cells in a tumor mass
[3-5]. CD133 is a transmembrane cell-surface glycoprotein. Cells
expressing this marker comprise from 3.5% to 46% of the total cells
in a tumor and are more frequent in tumors with a higher grade
of malignancy [6]. CD133+ cells repair DNA damage significantly
faster than CD133- cells [7]. Nestin is an intermediate filament
protein playing an important role in organogenesis, cell metabolism
and cytoskeletal organization. It may be re-expressed in neoplastic
transformation [8]. βIII-tubulin is one of the two globular proteins which form microtubules in combination with α-tubulin. This
protein is predominantly expressed in neurons and is involved in
neurogenesis. Overexpression of βIII-tubulin has been reported in
various cancers, including GBM [9].
GFAP is an intermediate filament protein. Its expression depends on the maturity of tumor cells. Therefore, primitive glioma cells may fail to exhibit reactivity with antibodies detecting the antigen, while mature cells are characterized by a high expression of GFAP [10]. Oct-4 and Nanog transcription factors are necessary for keeping stem cells in the state of pluripotency. Clinical studies have demonstrated the expression of Oct-4 and Nanog in a variety of cancers, including GBM [11-14]. Olig2, in turn, a transcription factor, whose expression is largely limited to the CNS, where it acts as a both anti-and pro-neurogenic agent, depending on the degree of cell development. It is understood that Olig2 is the most specific marker of GBM stem cells [15]. Rex is a marker of pluripotency of embryonic cells. Its expression decreases with the degree of cell differentiation [4]. A number of stem cell markers, including cancer and specifically GBM, have been identified and well described in literature. Previous research has revealed a relationship between some GBM stem cell markers and clinical features of cancer, such as a response to treatment and survival. However, no attempts have been made to determine a relation between the expression of stem cell markers in GBM and morphological features of this tumor.
Material and Methods
The Clinical Material
The study involved material containing cancer tissue collected during a routine neurosurgical treatment of 21 patients of both genders (10 women, 11 men) aged 30 to 77 years (median 56 years). The study protocol did not include another procedure during surgery or collection of more material than necessary for tumor removal. Nervous tissue comprising cancer tissue was frozen directly after removal in liquid nitrogen and frozen at -80°C. Once the diagnosis was confirmed by histopathological examination, detection of stem/progenitor cells was performed with the use of molecular assessment.
Laboratory Methods
Preparations for Histopathological Evaluation: The material
collected during surgery was fixed in a buffered formalin solution
for up to 24 hours, then dehydrated in acetone, put in xylene and
embedded in paraffin. Analysis was conducted on paraffin sections
of 4μm, stained with hematoxylin-eosin. In the assessment of GBM
histological features, the following were considered:
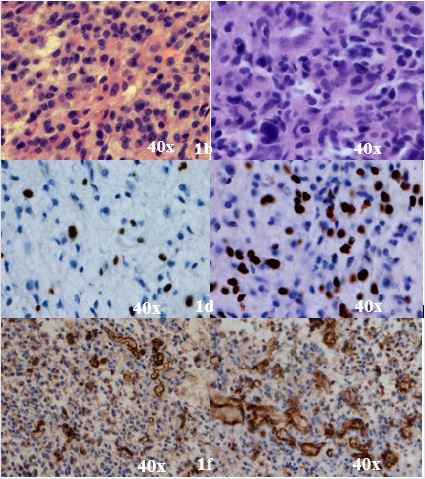
a) Nuclear pleomorphism (size, shape and hyperchromasia
of nuclei): relatively small vs. evident – Figures 1a & 1b; in four
consecutive fields of view in the hot spot area, magnification
40×
b) Ki-67 labeling index: up to 15% vs over 15% - Figures
1c & 1d;in four consecutive fields of view in the hot spot area,
magnification 40×
c) Endothelial proliferation: thin-walled vessels vs vessels
with marked proliferating endothelium – Figures 1e & 1f; in four
consecutive fields of view in the hot spot area, magnification
40×
Figure 1: Relatively small (1a) and evident (1b) pleomorphism within the GBM. Examples of proliferation activity within single specimen taken from GBM with Ki-67 labeling index < 15% (1c) and >15% (1d). Predominance of thin-walled vessels (1e) vs predominance of vessels with marked endothelium proliferation (1f).
Isolation of total RNA and Quantitative Reverse Transcription Reaction (qRT-PCR): Total RNA obtained from tumor tissue fragment was isolated using TRIzol® kit plus RNA Purification Kit (Life Technologies, USA). 0,5 g of RNA was subjected to reverse transcription process using a set of First Strand cDNA Synthesis Kit (Thermo Scientific, USA). Analysis of expression of target genes and a housekeeping gene β-actin was performed using the apparatus CFX96 Real Time System (Bio-Rad, USA). In the reaction, we used primers specific for the tested genes, the cDNA template and a ‘mix’ containing SYBR Green - iQ™ SYBR® Green Supermix (Bio-Rad, USA). Relative quantification of mRNA expression of genes tested was calculated using the comparative Ct method. The primers designed for qRT-PCR.
Statistical Methods
There were two main types of variables assessed: measurement variables and qualitative variables. The results were presented as mean and standard deviation. Data were compared between groups using the nonparametric Mann–Whitney U-test for continuous variables. All statistical tests were two-tailed, and p < 0.05 was considered to indicate statistical significance. All statistical analysis was performed with STATISTICA 12 software.
Results
a) Nuclear pleomorphism and the expression of cancer
stem cell markers: We found significantly higher expression
of the markers of CD133 (p = 0,0007) and Nestin (p = 0,0216)
in patients with evident nuclear pleomorphism in hotspot
regions.
b) Ki-67 labeling index and expression of cancer stem cell
markers: A significantly higher expression of CD133, Nestin
and Olig2 were found in patients with a Ki-67 labeling index
over 15% (respectively p = 0.0033, p = 0.0101 and p = 0.0066).
c) Endothelial proliferation and expression of cancer stem
cell markers: In the hotspot regions, there were no significant
differences between endothelial proliferation and the
expression of cancer stem cell markers.
Discussion
In conclusion it should be emphasized that despite a relatively
small number of patients in this study we were able to identify cells
in the GBM tissue which, using appropriate markers of cancer stem
cells, it can be assumed with high probability that these are GBM
stem cells. They coexisted significant with the pleomorphism and
proliferative index of this tumor. The cancer stem cells are most
likely responsible, at least in part, for the recurrence of tumor and
shorter survival. This hypothesis is supported by experimental
results, which show that cancer stem cells are characterized by high
activity of membrane proteins (ABC transporters) and increased
ability to repair DNA, also after radiotherapy [16-18,4,19]. In view
of the above, the present study compared the expression of cancer
stem cell markers with selected histopathological features of
GBM, such as nuclear pleomorphism, pathological proliferation of
endothelium including the formation of glomerular-shaped vessels
and tumor proliferative index. Significantly higher expression
of CD133 and Nestin were found in GBM with evident nuclear
pleomorphism. Such a role of CD133 protein may be promoted by
its ability to quickly repair damaged DNA [7].
Here we would like to pinpoint one of the mechanisms that
most likely contributes to the resistance of stem cells in the tumor
to the currently available treatment. GBM has features that play
an important role in tumor progression, such as invasiveness and
the ability to create specific vascularity. This allows cancer cells to
adapt to the anoxemic environment.
Persano et al. [26] showed that poorly differentiated cancer
stem cells of glioblastomas are located mainly in the layers of weakly
oxygenated cells, while more differentiated cancer cells are found
in better vascularized regions, and therefore, at least theoretically,
better supplied with oxygen and better nourished. Importantly,
cytostatics, including temozolomide, have a much worse reach into
areas with less blood.
Acknowledgement
We would like to thank to the Neurosurgery Department Pomeranian Medical University for their helpful assistance. This work was supported by Minister of Science and Higher Education Grants.
References
- Louis DN, Perry A, Reifenberger G, von Deimling, Figarella Branger D, et al. (2016) The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol 131(6): 803-820.
- Schwartzbaum JA, Fisher JL, Aldape KD, Wrensch M (2006) Epidemiology and molecular pathology of glioma. Nat Clin Pract Neurol 2(9): 494-503.
- Bradshaw A, Wickremsekera A, Tan ST, Peng L, Davis PF, et al. (2016) Cancer Stem Cell Hierarchy in Glioblastoma Multiforme. Front Surg 3(21).
- Kim BS, Kang KS, Choi JI, Jung JS, Im YB, et al. (2011) Knockdown of the potential cancer stem-like cell marker Rex-1 improves chemotherapeutic effects in gliomas. Hum Gene Ther 22(12): 1551-1562.
- Sampetrean O, Saya H (2013) Characteristics of glioma stem cells. Brain Tumor Pathol 30(4): 209-214.
- Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, et al. (2004) Identification of human brain tumour initiating cells. Nature 432(7015): 396-401.
- Bao S, Wu Q, McLendon RE, Hao Y, Shi Q, et al. (2006) Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature 444(7120): 756-760.
- Arai H, Ikota H, Sugawara K, Nobusawa S, Hirato J, et al. (2012) Nestin expression in brain tumors: its utility for pathological diagnosis and correlation with the prognosis of high-grade gliomas. Brain Tumor Pathol 29(3): 160-167.
- Masoumi S, Harisankar A, Gracias A, Bachinger F1, Fufa T, et al. (2016) Understanding cytoskeleton regulators in glioblastoma multiforme for therapy design. Drug Des Devel Ther 10: 2881-2897.
- Mei X, Chen YS, Chen FR, Xi SY, Chen ZP (2017) Glioblastoma stem cell differentiation into endothelial cells evidenced through live-cell imaging. Neuro Oncol 19(8): 1109-1118.
- Chang CC, Shieh GS, Wu P, Lin CC, Shiau AL, et al. (2008) Oct3/4 expression reflects tumor progression and regulates motility of bladder cancer cells. Cancer Res 68(15): 6281-6291.
- Chen D (2015) Tumor formation and drug resistance properties of human glioblastoma side population cells. Mol Med Rep 11(6): 4309-4314.
- Higgins DM, Wang R, Milligan B, Chroeder M, Carlson B, et al. (2013) Brain tumor stem cell multipotency correlates with nanog expression and extent of passaging in human glioblastoma xenografts. Oncotarget 4(5): 792-801.
- Schoenhals M, Kassambara A, De Vos J, Hose D, Moreaux J, et al. (2009) Embryonic stem cell markers expression in cancers. Biochem Biophys Res Commun. 2009; 383(2): 157-162.
- Trepant AL, Bouchart C, Rorive S, Sauvage S, Decaestecker C, et al. (2015) Identification of OLIG2 as the most specific glioblastoma stem cell marker starting from comparative analysis of data from similar DNA chip microarray platforms. Tumour Biol 36(3): 1943-1953.
- Dean M, Fojo T, Bates S (2005) Tumour stem cells and drug resistance. Nat Rev Cancer 5(4): 275-284.
- Donnenberg VS, Donnenberg AD (2005) Multiple drug resistance in cancer revisited: the cancer stem cell hypothesis. J Clin Pharmacol 45(8): 872-877.
- Khong Bee Kang, Congju Zhu, Yin Ling Wong, Qiuhan Gao, Albert Ty, et al. (2012) Gefitinib radiosensitizes stem-like glioma cells: inhibition of epidermal growth factor receptor-Akt-DNA-PK signaling, accompanied by inhibition of DNA double-strand break repair. Int J Radiat Oncol Biol Phys 83(1): e43-e52.
- Uribe D, Torres A, Rocha JD, Niechi I, Oyarzún C, et al. (2017) Multidrug resistance in glioblastoma stem-like cells: Role of the hypoxic microenvironment and adenosine signaling. Mol Aspects Med 55: 140-151.
- Tamagno I, Schiffer D (2006) Nestin expression in reactive astrocytes of human pathology. J Neurooncol 80(3): 227-233.
- Strojnik T, Røsland GV, Sakariassen PO, Kavalar R, Lah T, et al. (2007) Neural stem cell markers, nestin and musashi proteins, in the progression of human glioma: correlation of nestin with prognosis of patient survival. Surg Neurol 68(2): 133-143.
- Campbell JG, Miller DC, Cundiff DD, Feng Q, Litofsky NS (2015) Neural stem/progenitor cells react to non-glial cns neoplasms. Springerplus 4(53).
- De Bock K, Cauwenberghs S, Carmeliet P (2011) Vessel abnormalization: another hallmark of cancer? Molecular mechanisms and therapeutic implications. Curr Opin Genet Dev 21(1): 73-79.
- Zagzag D, Esencay M, Mendez O, Yee H, Smirnova I, et al. (2008) Hypoxia- and Vascular Endothelial Growth Factor-Induced Stromal Cell-Derived Factor - 1alpha/CXCR4 Expression in Glioblastomas one plausible explanation of Scherer's structures. Am J Pathol 173(2): 545-560.
- J M Heddleston, Z Li, J D Lathia, S Bao, A B Hjelmeland, et al. (2010) Hypoxia inducible factors in cancer stem cells. Br J Cancer 102: 789-795.
- Persano L, Rampazzo E, Basso G, Viola G, et al. (2013) Glioblastoma cancer stem cells: role of the microenvironment and therapeutic targeting. Biochem Pharmacol 85(5): 612-622.

 Research Article
Research Article