Abstract
In the current study, microwave-aided extraction (MAEX) has been used to extract mandarin leaves for its antioxidative phenolic and flavonoid ingredients. Satsuma mandarin leaves were used as research material. After the leaves were dried and ground, they were extracted by means of this advanced green tailor-designed liquid as well as conventional solvents (water, ethanol, methanol, ethyl acetate, acetonitrile and their water solutions). Total phenolic content (TPM) and total flavonoid content (TFM) content of the extracts were determined by UV-spectrophotometry. In addition, the antioxidant capacities of the extracts were measured by the inhibition activity of the extracts towards 2,2-diphenyl-1-picrylhydrazyl (DPPH) free radical. Deep eutectic solvents (DES) have been synthesized to extract the leaf samples. 3 DES containing citric acid and glycerol as hydrogen bond acceptor (HBA) and glycerol, dimethyl urea and methylimidazole hydrogen bond donor (HBD). Findings obtained from in vitro tests were evaluated statistically by using ANOVA.
Keywords: Deep Eutectic Liquids; Polyphenols; Antioxidant Activity; Extraction/ Separation; Microwave-Aided Extraction
Abbreviations: MAEX: Microwave-Aided Extraction; TPM: Total Phenolic Content; TFM: Total Flavonoid Content; DPPH: 2,2-diphenyl-1-picrylhydrazyl; DES: Deep Eutectic Solvents; HBA: Hydrogen Bond Acceptor; HBD: Hydrogen Bond Donor
Introduction
Citrus is a collection of citrus fruit tree species with high economic value such as orange, mandarin, grapefruit, bergamot and lemon. Turkey has a significant potential considering the citrus producing countries in the Mediterranean Basin. Therefore, Turkish citrus fruit products are the locomotive of processed fruit and vegetables export in Turkey [1]. Orange has the largest share in total citrus production with a ratio of 51.6%, followed by mandarin (25.5%), lemon (18.8%) and bitter orange (0.3%) [2]. On the other hand, the relevant crops, whose health benefits have been proven by numerous studies, also produce a large amount of waste such as peel, pomace, leaf and seeds. Furthermore, those waste byproducts contain numerous bioactive ingredients [3]. Hence, it is a great value to recover the biologically active ingredients from the concerned bio-wastes. Microwave-aided extraction (MAEX) is one of the advanced technologies for the recovery of high added value compounds from a complex environment containing so many ingredients such as protein, carbohydrate, lipids, pigments, waxes and more [4].
The advantage of microwave heating is the breakage of weak hydrogen bonds by dipole rotation of molecules. Furthermore, these dissolved ions in the medium facilitate the dissolution of the solute by increasing the penetration of the solvent into the solid material. Unlike conventional contact heat transfer methods, microwaves heat the entire sample at the same time. In this method, it is possible to heat quickly by using microwave energy [5]. On the other hand, design of environmentally friendly solvents has had a strategic preface in the framework of green technologies. The first purpose of the present this study is to synthesize these original solvents (DES), which are also considered as design products. In the second step, such novel solvents have been used to obtain phenolic antioxidants from citrus bio-waste through MAEX. Finally, the findings of DES have been compared to those of traditionally used solvents for control reasons.
Materials and Methods
Materials
Mandarin leaves used in the current study were obtained from the Western Mediterranean Agricultural Research Institute (BATEM). After drying at room temperature, it was stored in a dark and closed conditions. Ethanol, methanol, hexane, citric acid, and glycerol were from Merck (Darmstadt, Germany), while 2,2-diphenyl-1-picrylhydrazyl (DPPH), Folin-Cioacalteu, Na2CO3, methyimidazole and dimethylurea were of Sigma-Aldrich (St. Louis, MO, USA).
Preparation of DES
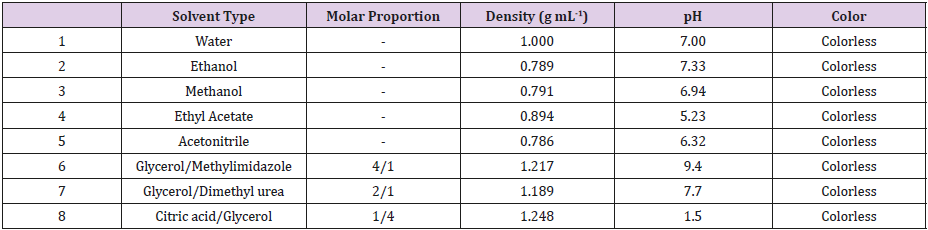
DES is a combination of a hydrogen bond acceptor (HBA) and a hydrogen bond donor (HBD). In this study, the DES combinations were prepared by using a heater assisted by an agitation at 80 ºC [6]. Table 1 shows the physical properties of the solvent systems. A pH meter (Metler Toledo, S220-K Seven Compact Schwerzebbach, Switzerland) and density meter (Anton Paar 4500, Graz, Austria) were applied for the measurements of pH and density.
Table 1: Physical properties used in the solvent systems for the extraction of mandarin leaves through microwave-aided extraction.
Procedure for MAEX
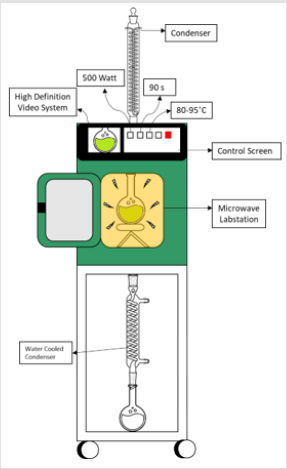
Figure 2: Mandarin leaf extracts obtained by several solvent systems through microwave-aided extraction.
This process was performed with Milestone NEOS-GR model microwave oven (Figure 1) under 500 W for 90 sec based on the preliminary experiments. Subsequently, the extracts obtained by each solvent system were centrifuged at 5000 rpm for 25 minutes in a centrifuge (Nüve, CN 180). The obtained extracts (Figure 2) were decanted and stored in cold and darkness. Each sample was passed through a 0.45 μm regenerated cellulose filter with a syringe and transferred to 1.2 mL vials before proceeding to the in vitro tests.2.4. In vitro tests for bioactive properties Total phenolic (TPM) and flavonoid (TFM) materials in the extracts were analyzed spectrophotometrically (PG Instruments, T60/Leicestershire, Leicester, England) at 765 nm [7] and 510 nm [8], respectively. The results were given as mg gallic acid (mg-GAE/g-DS) and catechin (mg-CE/g-DS) equivalents per gram dried leaf sample. As for antioxidant activity, inhibition of the free radical (DPPH) was measured at at 517 nm [9]. The antioxidant activity of the samples was described as inhibition of the DPPH radical (% inhibition) as explained in our previous paper [10].
Statistical Analysis
In order to analyse the findings (the means of three replicates) of the in vitro tests statistically, Tukey’s test was used as analysis of variance (ANOVA) statistical testing by means of InStat software (GraphPad, San Diego, CA, USA).
Results and Discussion
Comparison of the Performances of the Solvent Systems
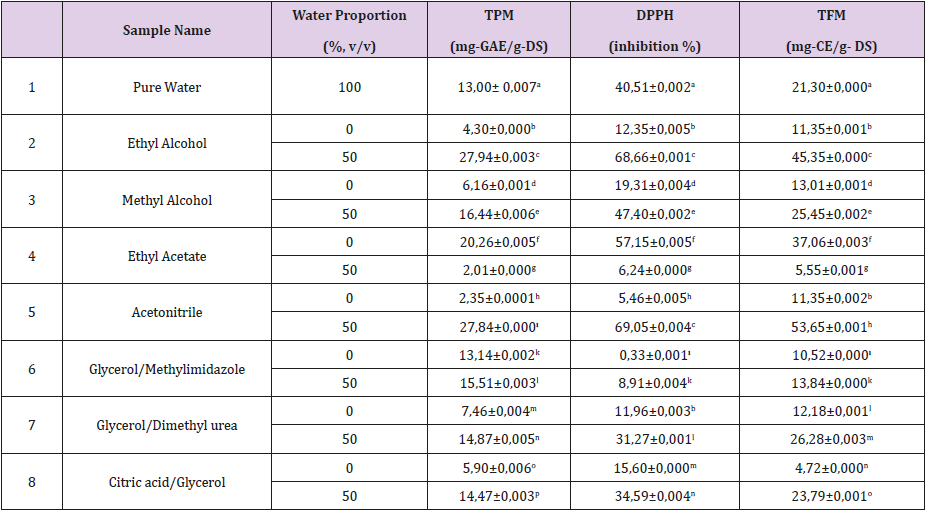
Table 2: Effects of solvent type on the bioactive contents of mandarin leaf extract obtained by microwave-aided extraction*.
Note: *Data are given as the mean (n = 3) ± standard deviation. Lines not sharing a common letter (a, b, c and d) indicate significant differences at p < 0.001
Table 2 demonstrates the phenolic and flavonoid ingredients and antioxidant activity of the mandarin leaf extracts obtained by MAEX. TPM changed between 2,01 and 27, 94 mg-GAE/g-DS, whereas TFM varied from 5,55 to 53,65 mg-CE/g-DS in the extracts obtained by several solvent systems. Phenolic contents of each solvent were statistically different at p < 0.001. As for TFM, pure ethanol and acetonitrile extracts produced similar values at p > 0.05. The poorest yields of TPM and TFM were obtained by ethyl acetate-water solution, whilst the greatest TPM and TFM were achieved by ethanol-water and acetonitrile-water solutions, respectively. On the other hand, the highest antioxidant activity was attained by ethanol-water and acetonitrile-water solutions (statistically the same at p > 0.05). Glycerol/Methylimidazole combination as DES showed relatively low activity against the selected free radical (Table 2).
Effect of Water on the Performances of the Solvent Systems
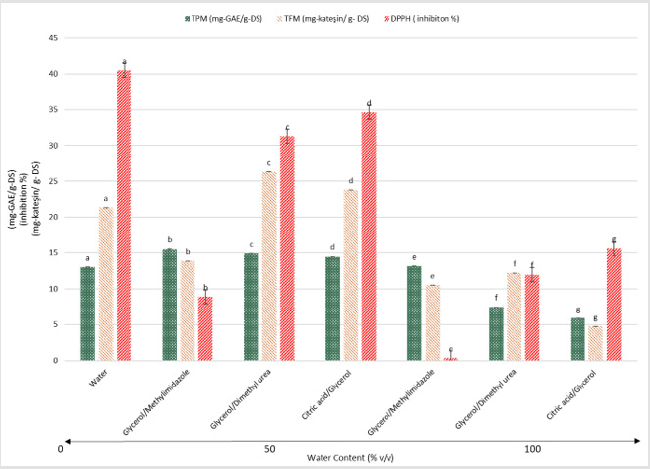
Figure 3 demonstrates the water effect in the extractant systems. Addition of 50% water into the selected solvents increased the yields of three dependent variables regularly, except for ethyl acetate. In the ethyl acetate extract, there was almost 10 times decrease in bioactive properties when 50% of water (v/v) was added into the solvent. The highest rise was observed in the acetonitrile system, where an approximately 14, 13 and 5 times increase in TPM, inhibition power and TFM yields were achieved. It was followed by ethanol system. Regarding DES systems, there was a slight increase in glycerol/methylimidazole liquid, which is an alkaline medium (Table 1). Glycerol/dimethyl urea (neutral medium) and citric acid/glycerol (acidic medium) combinations had almost sim ilar tendency towards the bioactivity properties. This output might be expected since acidic nature of the phenolic materials gave rise to degradation in alkaline extraction conditions [11].
Figure 3: Effect of water on the performances of the solvent. Data are given as the mean (n = 3) ± standard deviation.
Conclusion
Satsuma mandarin leaves were observed as potential raw material rich in bioactive ingredients with antioxidant power. The satisfactory correlations between the TPM and antioxidant activity (R2>0,73) and TFM and antioxidant activity (R2>0,88) demonstrate the contribution of phenolics and flavonoids to the antioxidant capacity of the leaves. Aqueous ethyl acetate solution (50%, v/v) was considered to be an insufficient solvent. Aqueous ethyl alcohol and acetonitrile solutions (50%, v/v) proved themselves as highly successful solvents. As for the deep eutectic solvents, the best performance was shown by acidic (citric acid/glycerol) and neutral (glycerol/dimethyl urea) ones. The last but not the least, further studies are needed to give a final declaration whether the findings are beneficial to human health.
References
- H Cetin Bedestenci KS Z (2000) Faculty of Agricultural Economics Kahramanmaras Handan HIT Zhou Z, Faculty of Agricultural Economics Department of Adana, CITRUS PRODUCTION AND FUTURE IN TURKEY, 2000.
- B Özkan, S Hatırlı, H Akçaöz, CF Karadeniz (2003) TURUNÇGİL FİYATLARININ ANALİZİ, Tarım Ekon. Derg 08: 37-49.
- S Şahin (2015) A novel technology for extraction of phenolic antioxidants from mandarin (Citrus deliciosa Tenore) leaves: Solvent-free microwave extraction. Korean J Chem Eng 32: 950-957.
- S Şahin, R Samli, ASB Tan, FJ Barba, F Chemat, et al. (2017) Solvent-Free Microwave-Assisted Extraction of Polyphenols from Olive Tree Leaves: Antioxidant and Antimicrobial Properties. Molecules 22: 1056.
- B Kaufmann, P Christen (2002) Recent extraction techniques for natural products: microwave-assisted extraction and pressurised solvent extraction. Phytochem Anal 13: 105-113.
- L Duan, LL Dou, L Guo, P Li, EH Liu (2016) Comprehensive Evaluation of Deep Eutectic Solvents in Extraction of Bioactive Natural Products.
- NSA Malik, JM Bradford (2008) Recovery and stability of oleuropein and other phenolic compounds during extraction and processing of olive (Olea europaea L.) leaves.
- S Sakanaka, Y Tachibana, Y Okada (2005) Preparation and antioxidant properties of extracts of Japanese persimmon leaf tea (kakinoha-cha). Food Chem 89: 569-575.
- S Şahin, R Şamli (2012) Optimization of olive leaf extract obtained by ultrasound-assisted extraction with response surface methodology. Ultrason Sonochem 20: 595-602.
- E Kurtulbaş, M Bilgin, S Şahin (2018) Assessment of lipid oxidation in cottonseed oil treated with phytonutrients: Kinetic and thermodynamic studies. Ind Crops Prod 124: 593-599.
- N Buchner, A Krumbein, S Rohn, LW Kroh (2006) Effect of thermal processing on the flavonols rutin and quercetin, Rapid Commun. Mass Spectrom 20: 3229-3235.

 Research Article
Research Article