Abstract
Manifesting much less mucosal irritating effect and higher efficiency than the currently used N-9, Tideglusib, a potent contraceptive candidate, exerts limited contraceptive efficacy in the water-soluble gels. Herein various new thiadiazolidine derivatives were synthesized and, for figuring out the essential groups that might be utilized to improve the in vivo efficacy of the lead, their sperm static/spermicidal effects were evaluated. By the analysis of the structure-activity relationship of tideglusib along with its derivatives, it was suggested that when the core ring was retained and the peripheral substituents were supplanted, the multiple derivatives have different levels of inhibition. And the naphthalene ring and benzene ring are non-essential.
Keywords:1,2,4-thiadiazolidine-3; 5-dione; Sperm; Spermicide; Contraceptive; Brake Activity
Introduction
estimation manifests that, by 2050, the world population is likely to reach 9.4 billion [1]. since the unmet contraceptive needs from 120 million couples, annually, result in 46 million abortions worldwide. And, unfortunately, no strategy could approach an ideal standard in contraception [2]. For the development of contraceptives, studies have been recently emphasized on inhibiting sperm motility, which might be a hopeful target [3]. If the vagina contains spermicides, the topical effective non-hormonal contraceptives, during sexual intercourse, make the vaginal sperm immobilized/deactivated/damaged, even killed, without causing systemic reactions [4].
However, by using the most common spermicide - nonoxynol-9 or other surfactant products, the detergent-type cytotoxic effect was currently proved to be the main disadvantage on vaginal cells [5]. Theoretically, vaginal spermicides with limited side effects have many advantages: they are female-controlled, inexpensive, safe and accessible [6]. And the inefficiency is the main limitation of spermicides. The pregnancy rate in the first year, approximately, ranged from 10% to 20%, which might be higher in typical use [7]. Although a variety of contraceptive methods have been developed, the acceptability of them is often restricted by the adverse side effects, failure rates or irreversibility [8]. Therefore, there is an urgent need to develop more effective, and customer-friendly contraceptives [9]. Only to find a better substitute for nonoxynol-9, could the main obstacle - to boost the protection and maintain a balance between contraceptive effects and vaginal environment, be overcame [10].
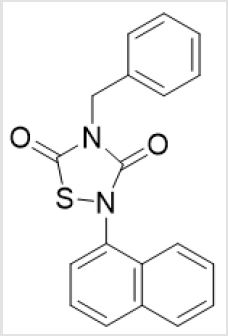
Unaffected by the acceptability [11], spermicide is deemed as a route of the administration for contraception for female, especially perimenopausal [12] and lactating women [13]. Based on the omics study of post-translational modification, the inhibitor was used to adjust the human sperm motility parameters in vitro. By screening molecules from the small compound library, it was found that the compounds have the ability to inhibit sperm motility [14]. Moreover, it was manifested that tideglusib had more efficient and lower cytotoxicity (mucosal damage) than N-9. Among them, there was a tideglusib, systematically named 4-benzyl-2-(naphthalen-1-yl) -1,2,4-thiadiazolidine-3,5-dione, came into our sight. The center of it is a 1,2,4-thiadiazolidine-3,5-dione ring, and the other 1-naphthyl and benzyl groups are attached to two nitrogen atoms, respectively. In this paper, we intended to synthesize derivatives of this compound and test the sperm braking/killing activity by analyzing the essential groups of the brake sperm through structure-effects, then we optimized the structure for subsequent transformation to achieve a good braking effect and laid the foundation to obtaining high-effective in vivo contraceptive effects in animals (Figure 1).
Investigations, Results and Discussion
In this study, since tideglusib is a well-known phase II clinical
drug with known molecular structure, we used it as a lead
compound to synthesize a series of derivatives for screening for
excellent immobilizing performance (high water solubility and low
toxicity). Based on the structure-activity relationship, we intended
to determine the active group of it. In vitro experiments manifested
that tideglusib had a strong immobilizing effect on human sperm
(MEC concentration is about 10μM), while the vaginal/mucosal
toxicity was lower than N-9, which meant that it might have potent
drug-forming properties and could be a potential N-9 substitutes
and non-hormonal contraceptives, deserving deep research and
development. However, the poor solubility resulted in a decrease
of contraceptive effect, which suggested us to improve the water
solubility. In view of the unclear mechanism, we attempted to design
and synthesize a series of In addition, we explored the potential of
1,2,4-thiadiazolidin-3,5-dione derivatives and their effects against
human sperm as a novel spermostatic.
To evaluate the antifertility activity, various new derivatives
have been synthesized. In summary, a novel series of
1,2,4-Thiadiazolidin-3,5-dione derivatives have been synthesized
and biologically evaluated as an innovated spermostatic. The
bio-experimental results showed the differences among newly
synthesized derivatives in the ability of braking spermatozoa,
according to which we could grade the compounds, based on the
final observation index of all-sperm-braked in the culture system
(96-well plate). After the working concentration of the compound
reached 500μM, with the incubation times coming up to 20 min, if
the sperm motility showed no change compared with the control
group (no compound added), the compound should be considered
as probably invalid, defined as “null”. The activity level of the
original tideglusib was defined as “*****”, and the number of “*”
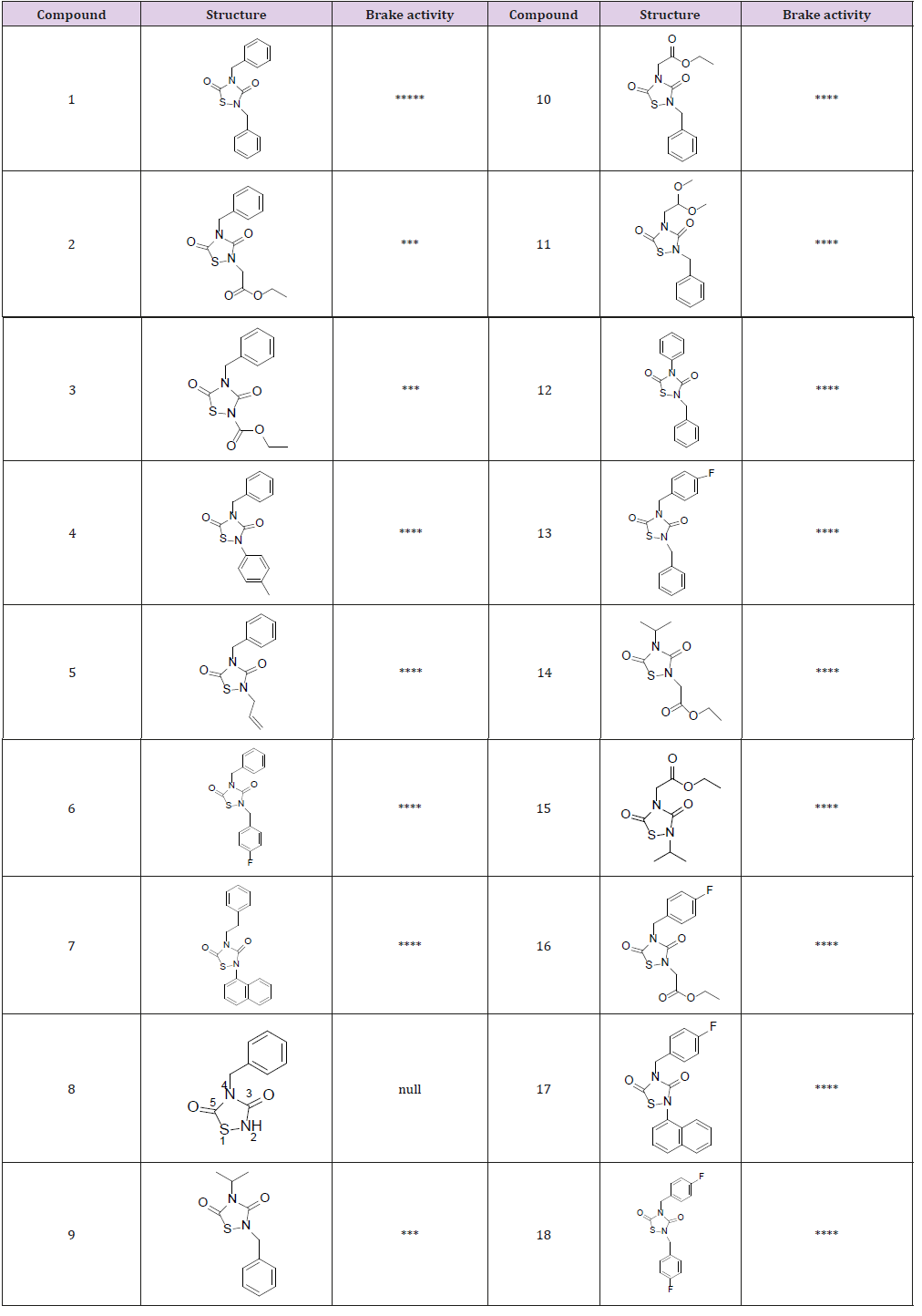
represented the degree of braking activity. As shown in Table 1, all
of the 17 thiadiazolidinone derivatives have inhibitory effects on
sperm motility, except for compound 8, in which 2-N was connected
with H atom. By studying the structure-activity relationship among
tideglusib and its chemical derivatives, it was found that both
the naphthalene ring and the benzene ring were brake-active
non-essential groups. When the core ring was retained and the
peripheral substituents were supplanted, the multiple derivatives
have different levels of inhibition. But when the 2-N was connected
with H, the compound could not inhibit sperm activity.
Table 1: Structural of the compound and sperm braking ability. The number of “*” stands for the ability to brake sperm.
Experimental
Collection of Fresh Semen Specimens
In the period of January 2017 to December 2018, the required specimens were collected with masturbation method from the volunteers, 25-35 years old, after a abstinence about 3-7 days. Then the specimens were treated in the outpatient department of Shanghai Family Planning Research Institute Hospital or the Reproductive Center of Zhongshan Hospital of Fudan University. Semen specimen requirements: liquefaction time ≤ 0.5 h, semen volume ≥ 1.5 mL, pH value 7.2-8.0, white blood cells <1 × 106/ mL, sperm density ≥ 15 × 106 / mL, sperm motility meets (a + b) Grade sperm ≥ 50% or grade a sperm ≥ 25%, both seminal plasma and serum anti-sperm antibodies were negative. The study was approved by the Medical Ethics Committee of the Shanghai Institute of Planned Parenthood (project name: research on the mechanism of action of the new sperm brake agent tideglusib and its chemical derivatives, approval number: PJ2018-24) and the required semen all specimens received informed consent from the donors and all specimens were boiled at high temperature after the end of the experiment.
Acquisition of Human High Vitality Sperm
According to the instructions of the 5th edition of the WHO Human Semen Examination and Handling Laboratory Manual, human spermatozoa are collected by the direct upstream method of sperm. First, add 1 mL of BWW medium to a 15 mL centrifuge tube, then slowly mix the well-mixed semen samples into the bottom of the centrifuge tube, 0.5 mL per tube, tilt the tube 45°, and place at 37°C, 5% CO2. Place in the incubator for 45 min-1 h. Take 0.5 mL of the mixture in the upper layer to obtain high activity sperm, and adjust the sperm concentration to 10~20×106/ mL in BWW medium for use.
Determination of The Effect of Tideglusib Derivatives on Sperm Braking
The tideglusib and its related derivatives were diluted with BWW at a maximum concentration of 500μM, 50 μL per well. The 96-well plate containing the compound was placed in a 37 °C, 5% CO2 incubator for preheating, 0.5 h. High-activity sperm were collected according to the method shown in 3.1. Mix 50μL of the sperm suspension with the compound in the 96-well plate and quickly press the stopwatch for 20 s. Solvent DMSO was the negative control and N-9 was the positive control. Under the microscope (400×), the compound was able to break all the sperm at this concentration, thereby measuring the minimum effective concentration (MEC) of the drug required to lose the motility of all sperm within 20s. The experiment was repeated at least three times to obtain an average MEC.
Chemistry
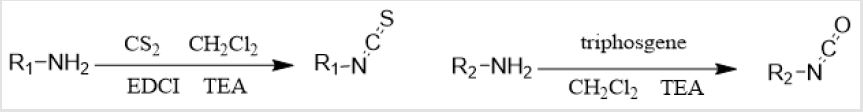
All solvents and reagents were purchased from commercial suppliers and were used without further purification unless otherwise stated. 1H NMR and 13C NMR spectra were recorded on a Bruker 400 MHz or 600MHz spectrometer. Chemical shifts were reported as δ values relative to the internal standard TMS (Me4Si). High Resolution MS spectra were obtained on a JEOL AccuTOF-MS instrument. Column chromatography separations were performed on silica gel (200–300 mesh, Qingdao Ocean Chemical Co, Ltd, Qingdao, P.R. China) (Scheme 1).
Scheme 1: Synthesis of the corresponding isocyanates and isothiocyanates from primary amines containing different substituents. (Some isocyanates were purchased commercially, and some were synthesized in the laboratory. The synthetic route is shown below in Scheme 1).
Synthetic Methods
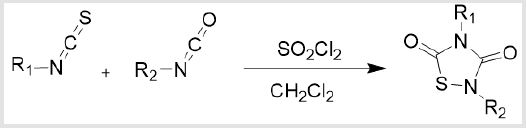
General Procedure for the Synthesis of 1,2,4-Thiadiazolidine- 3,5-dione: A 50mL round botton flask was charged with isocyanate (5mmol), isothiocyanate (5mmol), Hexane (5mL) and CH2Cl2 (5mL), cooled to 0°C. Sulfuryl chloride (5mmol) was added slowly in the solution. The mixture was allowed to be stirred in room temperature overnight. The solution was opened to the air for 30 minutes, fully reacted with water, then removed solvent under reduced pressure. The residue was diluted with ethyl acetate (30mL) and saturated NaHCO3 aqueous solution (10mL), and the aqueous phase was extracted with ethyl acetate (20mL) three times. The combined organic phase was dried (Na2SO4) and removed by rotary evaporation. Flash chromatography (From PE to PE:EA=8:2) was used to purify the crude reaction mixture [15].
a) Synthetic Isothiocyanate: Primary amine (10mmol) was dissolved in CH2Cl2 (10mL), then TEA (10 mmol) was added and the mixture reacted for 30 minutes, the solution was cooled to 0℃, CS2sub> (50 mmol) and EDCI (10mmol) was added at room temperature, stirred overnight. Solvent was removed under reduced pressure. The residue was diluted with ethyl acetate (30mL) and saturated NaHCO3 aqueous solution (10 mL), the aqueous phase was extracted with ethyl acetate (20mL) for three times. The combined organic phase was dried (Na2SO4) and removed by rotary evaporation. Flash chromatography (petroleum ether) was used to purify the crude reaction mixture (Scheme 2).
Scheme 2: Isocyanates and isothiocyanates having different substituents are prepared by using dichloromethane and cyclohexane as reaction solvents, adding sulfuryl chloride under ice bath, and stirring at room temperature overnight to synthesize thiadiazoledione derivatives with different substituents.
b) Synthetic Isocyanate: A 50mL round botton flask was charged with primary amine (10mmol), CH2Cl2 (20mL) and TEA (20mmol). In another 50mL round botton flask, triphosgene (3.3mmol) was dissolved in CH2Cl2 (10mL). Then the solution was cooled to 0℃. The first mixture, which included primary amine, TEA and CH2Cl2, was added solwly to the second solution inclueding triphosgene and CH2Cl2. The solution reacted for 30 minutes under ice bath, then stirred overnight at room temperature. The mixture was washed twice with cold 0.1 M hydrochloric acid aqueous solution (10mL) and cold saturated NaCl aqueous solution (10mL) (Scheme 3). The combined organic phase was dried (MgSO4), filtered, and removed by rotary evaporation to obtain the isocyanate [16].
Ethyl 2-(4-benzyl-3,5-dioxo-1,2,4-thiadiazolidin-2-yl) acetate (CAS: 1018473-58-9): Compound 2 was prepared by following the above general procedure. Yield: 52.2%. 1H NMR (400 MHz, CDCl3) δ 7.41 (d, J = 7.2 Hz, 2H), 7.34 – 7.24 (m, 3H), 4.81 (s, 2H), 4.28 (s, 2H), 4.19 (q, J = 7.1 Hz, 2H), 1.23 (t, J = 7.2 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 167.12, 166.02, 153.83, 135.04, 128.74, 128.66, 128.30, 62.17, 46.08, 45.62, 14.06. HR ESI MS: m/z calcd for C13H- 15N2O4S [M+H] +, 295.0747, found, 295.0747.
Ethyl 4-benzyl-3,5-dioxo-1,2,4-thiadiazolidine-2-carboxylate (CAS: 1622394-95-9): Compound 3 was prepared by following the above general procedure. Yield: 41.4%. 1H NMR (400 MHz, CDCl3) δ 7.49 – 7.30 (m, 5H), 4.84 (s, 2H), 4.41 (q, J = 7.1 Hz, 2H), 1.39 (t, J = 7.1 Hz, 3H). 13C NMR (151 MHz, CDCl3) δ 164.14, 148.31, 148.23, 134.34, 129.21, 128.84, 128.63, 65.51, 45.96, 14.21. HR ESI MS: m/z calcd for C12H13N2O4S [M+H] +, 281.0591, found, 281.0593.
4-benzyl-2-(p-tolyl)-1,2,4-thiadiazolidine-3,5-dione (CAS: 1352551-97-3): Compound 4 was prepared by following the above general procedure. Yield: 52.6%. 1H NMR (400 MHz, CDCl3) δ 7.50 (d, J = 6.5 Hz, 2H), 7.36 (m, 5H), 7.20 (d, J = 8.3 Hz, 2H), 4.91 (s, 2H), 2.35 (s, 3H). 13C NMR (151 MHz, CDCl3) δ 165.25, 151.11, 137.29, 135.09, 133.07, 130.06, 129.14, 128.78, 128.39, 123.70, 46.15, 21.01. HR ESI MS: m/z calcd for C16H15N2O2S [M+H] +, 299.0849, found, 299.0850.
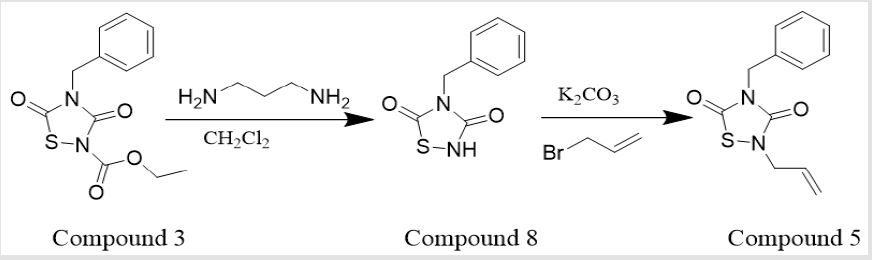
2-allyl-4-benzyl-1,2,4-thiadiazolidine-3,5-dione (CAS:1111871-39-6): The compound (0.5 mmol) and 3-bromopropene (0.5 mmol) were dissolved in acetonitrile (5 mL), then K2CO3 (1 mmol) was added and the mixture was stirred at room temperature for 4.5 hours [17]. The mixture was diluted with ethyl acetate (30 mL) and H2O (10 mL). The combined organic phase was dried (Na2SO4) and removed by rotary evaporation to obtain the compound 5. Yield: 84.3%. 1H NMR (400 MHz, CDCl3) δ 7.38 (m, 5H), 5.82 (m, 1H), 5.32 (s, 2H), 4.83 (s, 2H), 4.23 (d, J = 6.3 Hz, 2H). 13C NMR (151 MHz, CDCl3) δ 166.05, 152.87, 135.19, 130.99, 128.92, 128.74, 128.30, 120.80, 47.29, 45.97. HR ESI MS: m/z calcd for C12H13N2O2S [M+H] +, 249.0692, found, 249.0694.
4-benzyl-2-(4-fluorobenzyl)-1,2,4-thiadiazolidine-3,5-dione: Compound 6 was prepared by following the above general procedure. Yield: 54.4%. 1H NMR (400 MHz, CDCl3) δ 7.48 (d, J = 6.8 Hz, 2H), 7.37 (m, 3H), 7.33 – 7.29 (m, 2H), 7.09 (t, J = 8.6 Hz, 2H), 4.87 (s, 2H), 4.76 (s, 2H). 19F NMR (377 MHz, CDCl3) δ -112.47 (s). 13C NMR (101 MHz, CDCl3) δ 165.70, 162.94 (d, J = 248.2 Hz), 153.08, 135.13, 130.40 (d, J = 8.4 Hz), 130.31 (d, J = 3.3 Hz), 128.92, 128.77, 128.35, 116.09 (d, J = 21.7 Hz), 48.08, 46.07. HR ESI MS: m/z calcd for C16H14N2O2FS [M+H] +, 317.0755, found, 317.0757.
2-(naphthalen-1-yl)-4-phenethyl-1,2,4-thiadiazolidine- 3,5-dione: Compound 7 was prepared by following the above general procedure. Yield: 51.7%. 1H NMR (400 MHz, CDCl3) δ 7.93 (m, 2H), 7.64 – 7.47 (m, 5H), 7.40 – 7.27 (m, 5H), 4.10 (t, J = 8 Hz, 2H), 3.14 (t, J = 8 Hz, 2H). 13C NMR (101 MHz, CDCl3) δ 166.20, 152.23, 137.29, 134.59, 130.96, 130.53, 130.40, 129.13, 128.71, 128.68, 127.65, 127.29, 126.97, 126.91, 125.42, 122.29, 43.94, 33.58. HR ESI MS: m/z calcd for C220H17N2O2S [M+H] +, 349.1005, found, 349.1001.
4-benzyl-1,2,4-thiadiazolidine-3,5-dione (CAS: 26668- 32-6): The compound 3 (1.4 mmol) was dissolved in CH2Cl2 (10 mL), ethylenediamine (1 mL) was added and the mixture was allowed to be stirred at room temperature for 1.5 hours, then formic acid (4 mL) was added , the solvent was removed under reduced pressure. The residue was diluted with ethyl acetate (30 mL) and NH4Cl (10 mL), and the aqueous phase was extracted with ethyl acetate (20 mL). The combined organic phase was dried (Na2SO4) and removed by rotary evaporation to obtain the compound 8[17]. Yield: 58.9%. 1H NMR (400 MHz, CDCl3) δ 7.36 (m, 5H), 4.82 (s, 2H). 13C NMR (151 MHz, CDCl3) δ 167.76, 155.34, 134.86, 128.80, 128.72, 128.41, 45.58. HR ESI MS: m/z calcd for C9H9N2O2S [M+H] +, 209.0379, found, 209.0379.
2-benzyl-4-isopropyl-1,2,4-thiadiazolidine-3,5-dione: Compound 9 was prepared by following the above general procedure. Yield: 56.2%. 1H NMR (400 MHz, CDCl3) δ 7.35 (d, J = 6.0 Hz, 3H), 7.28 (d, J = 7.1 Hz, 2H), 4.73 (s, 2H), 4.55 (dt, J = 13.8, 6.9 Hz, 1H), 1.47 (d, J = 7.1 Hz, 6H). 13C NMR (101 MHz, CDCl3) δ 165.76, 153.12, 134.74, 129.02, 128.73, 128.48, 48.61, 48.19, 19.30. HR ESI MS: m/z calcd for C12sub> H15N2O2S [M+H] +, 251.0849, found, 251.0854.
Ethyl 2-(2-benzyl-3,5-dioxo-1,2,4-thiadiazolidin-4-yl) acetate: Compound 10 was prepared by following the above general procedure. Yield: 56.6%. 1H NMR (400 MHz, CDCl3) δ 7.44 – 7.28 (m, 5H), 4.82 (s, 2H), 4.43 (s, 2H), 4.26 (q, J = 7.2 Hz, 2H), 1.30 (t, J = 7.2 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 166.40, 165.70, 152.48, 134.26, 129.11, 128.90, 128.39, 62.16, 48.74, 42.71, 14.08. HR ESI MS: m/z calcd for C13H15N2O4S [M+H] +, 295.0747, found, 295.0746.
2-benzyl-4-(2,2-dimethoxyethyl)-1,2,4-thiadiazolidine- 3,5-dione: Compound 11 was prepared by following the above general procedure. Yield: 54.9%. 1H NMR (400 MHz, CDCl3) δ 7.37 (brs, 3H), 7.30 (m, 2H), 4.79 (brs, 3H), 3.83 (d, J = 5.6 Hz, 2H), 3.39 (s, 6H). 13C NMR (101 MHz, CDCl3) δ 165.96, 153.00, 134.39, 129.06, 128.85, 128.44, 99.30, 53.32, 48.69, 42.76. HR ESI MS: m/z calcd for C13H16N2O4NaS [M+Na] +, 319.0723, found, 319.0721.
2-benzyl-4-phenyl-1,2,4-thiadiazolidine-3,5-dione: Compound 12 was prepared by following the above general procedure. Yield: 59.6%. 1H NMR (400 MHz, CDCl3) δ 7.44 (m, 10H), 4.85 (s, 2H). 13C NMR (101 MHz, CDCl3) δ 165.47, 152.49, 134.39, 132.75, 129.41, 129.22, 129.17, 129.00, 128.71, 127.26, 49.01. HR ESI MS: m/z calcd for C15H13N2O2S [M+H] +, 285.0692, found, 285.0697.
2-benzyl-4-(4-fluorobenzyl)-1,2,4-thiadiazolidine-3,5-dione (CAS: 1055193-37-7): Compound 13 was prepared by following the above general procedure. Yield: 68.6%. 1H NMR (400 MHz, CDCl3) δ 7.45 (dd, J = 8.3, 5.5 Hz, 2H), 7.37 (m, 3H), 7.28 (m, 2H), 7.02 (t, J = 8.6 Hz, 2H), 4.81 (s, 2H), 4.76 (s, 2H). 19F NMR (377 MHz, CDCl3) δ -113.25 – -113.49 (m). 13C NMR (101 MHz, CDCl3) δ 165.88, 162.72 (d, J = 247.1 Hz), 153.01, 134.40, 131.10 (d, J = 3.3 Hz), 130.95 (d, J = 8.3 Hz), 129.10, 128.92, 128.52, 115.67 (d, J = 21.6 Hz), 48.80, 45.28. HR ESI MS: m/z calcd for C16H13N2O2FNaS [M+Na] +, 339.0574, found, 339.0576.
Ethyl 2-(4-isopropyl-3,5-dioxo-1,2,4-thiadiazolidin-2-yl) acetate: Compound 14 was prepared by following the above general procedure. Yield: 60.4%. 1H NMR (400 MHz, CDCl3) δ 4.55 (dt, J = 13.8, 6.9 Hz, 1H), 4.30 (s, 2H), 4.25 (q, J = 7.1 Hz, 2H), 1.49 (d, J = 6.9 Hz, 6H), 1.30 (t, J = 7.1 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 167.20, 165.92, 153.84, 62.05, 48.30, 45.54, 19.11, 14.02. HR ESI MS: m/z calcd for C9H15N2O4S [M+H] +, 247.0747, found, 247.0752.
Ethyl 2-(2-isopropyl-3,5-dioxo-1,2,4-thiadiazolidin-4-yl) acetate (CAS: 1018473-68-1): Compound 15 was prepared by following the above general procedure. Yield: 66.1%. 1H NMR (400 MHz, CDCl3) δ 4.74 – 4.65 (m, 1H), 4.39 (s, 2H), 4.24 (d, J = 7.1 Hz, 2H), 1.31 (dd, J = 6.9, 3.6 Hz, 9H). 13C NMR (101 MHz, CDCl3) δ 166.48, 166.11, 151.79, 62.08, 47.50, 42.44, 21.25, 14.07. HR ESI MS: m/z calcd for C9H14N2O4NaS [M+Na]+, 269.0567, found, 269.0571.
Ethyl 2-(4-(4-fluorobenzyl)-3,5-dioxo-1,2,4-thiadiazolidin- 2-yl) acetate (CAS: 1055193-62-8): Compound 16 was prepared by following the above general procedure. Yield: 52.2%. 1H NMR (400 MHz, CDCl3) δ 7.42 (m, 2H), 7.06 – 6.96 (m, 2H), 4.81 (s, 2H), 4.32 (s, 2H), 4.23 (q, J = 7.1 Hz, 2H), 1.26 (t, J = 6Hz, 3H). 19F NMR (377 MHz, CDCl3) δ -113.39 – -113.52 (m). 13C NMR (101 MHz, CDCl3) δ 167.01, 165.95, 162.69 (d, J = 247.1 Hz), 153.75, 130.86 (d, J = 3.3 Hz), 130.77 (d, J = 8.3 Hz), 115.65 (d, J = 21.6 Hz), 62.24, 45.63, 45.37, 14.05. HR ESI MS: m/z calcd for C13H- 13N2O4FNaS [M+Na] +, 335.0472, found, 335.0466.
4-(4-fluorobenzyl)-2-(naphthalen-1-yl)-1,2,4-thiadiazolidine- 3,5-dione: Compound 17 was prepared by following the above general procedure. Yield: 37.4%. 1H NMR (400 MHz, CDCl3) δ 7.97 (m, 2H), 7.81 – 7.75 (m, 1H), 7.64 – 7.51 (m, 6H), 7.09 (m, 2H), 4.98 (s, 2H). 19F NMR (377 MHz, CDCl3) δ -113.24 (s). 13C NMR (101 MHz, CDCl3) δ 166.16, 162.81 (d, J = 247.3 Hz), 152.35, 134.60, 131.23 (d, J = 8.3 Hz), 131.08 (d, J = 3.2 Hz), 130.89, 130.51, 130.42, 128.75, 127.71, 127.38, 127.02, 125.43, 122.16, 115.75 (d, J = 21.5 Hz), 45.65. HR ESI MS: m/z calcd for C19H14N2O2FS [M+H] +, 353.0755, found, 353.0743.
2,4-bis(4-fluorobenzyl)-1,2,4-thiadiazolidine-3,5-dione: Compound 18 was prepared by following the above general procedure. Yield: 46.6%. 1H NMR (400 MHz, CDCl3) δ 7.47 (m, 2H), 7.34 – 7.29 (m, 2H), 7.07 (m, 4H), 4.83 (s, 2H), 4.76 (s, 2H). 19F NMR (377 MHz, CDCl3) δ -112.36 (s), -113.34 (s). 13C NMR (101 MHz, CDCl3) δ 165.68, 164.07 (d, J = 24.9 Hz), 161.60 (d, J = 23.8 Hz), 152.95, 130.95 (d, J = 8.3 Hz), 130.41 (d, J = 8.4 Hz), 130.23 (d, J = 3.3 Hz), 116.11 (d, J = 21.7 Hz), 115.68 (d, J = 21.6 Hz), 48.08, 45.31. HR ESI MS: m/z calcd for C16H13N2O2F2S [M+H] +, 335.0660, found, 335.0655.
Acknowledgement
This work was supported by the Fundamental Research Funds for the Central Universities (NO.2232018D3-40) and the National Natural Science Foundation of China (NO. 81671508).
References
- Chakraborty D, Maity A, Jha T (2014) Spermicidal and Contraceptive Potential of Desgalactotigonin: A Prospective Alternative of Nonoxynol-9. [J] PLOS ONE.
- Hughes LM, Griffith R, Carey A (2009) The Spermostatic and Microbicidal Actions of Quinones and Maleimides: Toward a Dual-Purpose Contraceptive Agent. [J] Molecular Pharmacology 76(1): 113-124.
- Wang H, Chen XX, Wang LR (2010) AF-2364 is a prospective spermicide candidate. [J] Asian Journal of Andrology 12(3): 322-335.
- Iyer V, Poddar SS (2008) Update on nonoxynol-9 as vaginal spermicide. [J] The European Journal of Contraception and Reproductive Health Care 13(4): 339-350.
- Bharitkar YP, Banerjee M, Kumar S, Kuotsu K, Mondal NB, et al. (2013) Search for a potent microbicidal spermicide from the isolates of Shorea robusta resin. [J] Contraception 88(1): 133-140.
- Schreiber CA, Ratcliffe SJ, Sammel MD, Whittaker PG (2016) A self-assessment efficacy tool for spermicide contraceptive users. [J] American Journal of Obstetrics and Gynecology 214(2): 264.e1-264.e7.
- Raymond EG, Trussell J, Weaver MA, Reeves MF (2013) Estimating contraceptive efficacy: the case of spermicides. [J] Contraception 87(2): 134-137.
- Singh KK, Parmar S, Tatke PA (2012) Contraceptive efficacy and safety of HerbOshield? vaginal gel in rats. [J] Contraception 85(1): 122-127.
- Hughes LM, Griffith R, Carey A (2009) The Spermostatic and Microbicidal Actions of Quinones and Maleimides: Toward a Dual-Purpose Contraceptive Agent. [J] Molecular Pharmacology 76(1): 113-124.
- Grimes DA, Lopez LM, Raymond EG (2013) Spermicide used alone for contraception[J]. Cochrane Database of Systematic Reviews 12(4): CD005218.
- Raymond EG, Chen PL, Condon S (2005) Acceptability of five nonoxynol-9 spermicides[J]. Contraception 71(6): 438-442.
- Serfaty D (2017) Contraception during perimenopause: The spermicides option.[J]. Journal of Gynecology Obstetrics & Human Reproduction 46(3): 211.
- Serfaty D (2014) [Contraception in breastfeeding women: place for spermicides]. [J] Journal De Gynécologie Obstétrique Et Biologie De La Reproduction 44(1): 18-27.
- Zhiting Chen, Niyan Shu, Yuzhu Wang, Yiting Yang, Zhiyu Shao, et al. (2019) Tideglusib, a prospective alternative to nonoxynol-9 contraceptive, Contraception: X 1: 2590-1516.
- Turner EM, Blazer LL, Neubig RR (2012) Small Molecule Inhibitors of Regulators of G Protein Signaling (RGS) Proteins. [J] ACS medicinal chemistry letters 3(2): 146-150.
- Cha YF, Zhang S, Su H, Min Luo, Yong Huang, et al. (2015) l-Amino acid carbamate prodrugs of scutellarin: synthesis, physiochemical property, Caco-2 cell permeability, and in vitro anti-oxidative activity. [J] Medicinal Chemistry Research 24(5): 2238-2246.
- Wang Lei, Gong Zhaolong, Zhang, Zhengping (2015) Preparation of 1,2,4-thiadiazole-3,5-dione derivatives as inhibitors of glycogen synthase kinase-3β and acetylcholinesterase.

 Research Article
Research Article