Abstract
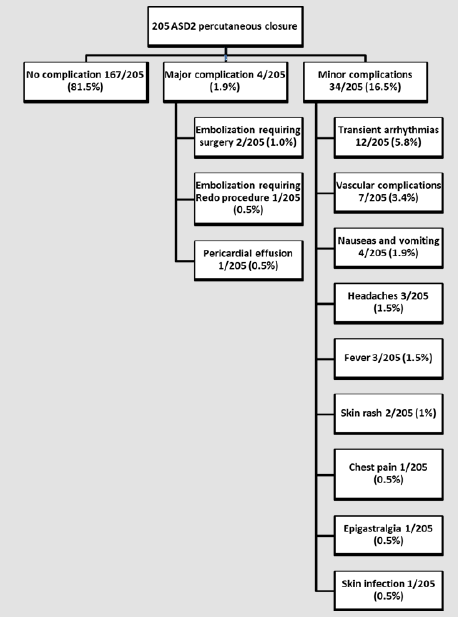
Over the past 20 years, closure of a secundum Atrial Septal Defect (ASD) has switched from a surgical approach to a transcatheter percutaneous approach. There are only few long-term studies on the efficacy and safety of percutaneous ASD closure in children. Data on all 220 children who admitted to the catheterization laboratory between 1999 and 2011 for attempted ASD closure were retrospectively reviewed in conjunction with a study performed at our institution to assess the results of surgical closure during the same study period. Of 220 patients, 205 underwent attempted ASD closure (mean age 7.96 years±4.15 years, mean weight 29.05Kg±17.0Kg), with effective closure in 203 patients (success rate: 92%). There were no deaths. There were no complications in 179 patients (88%). Major complications occurred in three patients (1.5%): two embolizations of the device (1%) requiring cardiac surgery to retrieve the device and close the ASD, and one patient (0.5%) with pericardial effusion requiring pericardial drainage. In hospital, minor transient complications were recorded in 22 patients (10.7%): 12 arrhythmias (5.8%); seven vascular complications (3.4%); four cases of nausea and vomiting (1.9%); three patients with headache (1.5%); two with skin rash (1%); one with chest pain (0.5%); one with epigastralgia (0.5%); and one skin infection at the puncture site requiring local treatment and antibiotics (0.5%).
There were no major complications during long-term follow-up, while minor and transient complications were reported during the first month in 17 patients (8.3%) and during long-term follow-up in 27 patients (13.2%). These complications were not clearly related to the procedure, with a reassuring medical work-up in all cases: chest pain in 14 patients (6.8%); palpitations in 12 (5.8%); headache and dizziness in seven (3.4%); epistaxis in one (0.5%); and mild pericardial effusion not requiring treatment in three (1.5%). Six months after the procedure, 12 patients (5.8%) had a residual shunt of more than 4mm in diameter on echo, with a pulmonary-to-systemic flow ratio on Doppler of less than 1.5 in all cases. In line with the published data, our series reveals that the procedure is safe, well tolerated, and effective in the majority of children, with no significant complications for 8–20 years after percutaneous ASD closure. In conjunction with the study performed at our institution to assess the results of surgical closure during the same period, percutaneous ASD closure remains an appropriate alternative to surgery in children.
Keywords: Atrial Septal Defect; Catheterization; Complications; Children
Abbreviations:
ASD: Atrial Septal Defect; ASO: Amplatzer Septal Occluder; A-V: Auriculo ventricular; EKG: Electrocardiogram; LMWH: Low Molecular Weight Heparin; TEE: Transesophageal Echocardiography; TTE: Transthoracic Echocardiography
Introduction
Atrial Septal Defect (ASD) accounts for 8–10% of congenital heart disorders [1,2], with ostium secundum ASD (ASD2) representing approximately 75% of all ASDs. Since the first percutaneous attempt of ASD closure in 1976 by King [3], major technological and technical advances have enabled the transcatheter approach to become the first-line treatment for ASD2 closure. The objective of this study was to review the results of the percutaneous transcatheter approach performed as a first-line treatment in 65% of children with isolated ASD2 at our institution. In conjunction with a study performed to assess the results of ASD2 surgical closure, this review provides comparative data between the two approaches.
Material and Methods
Patients and Methods
We retrospectively studied 220 children (≤18 years) who had undergone attempted closure of ASD2 by catheterization between 1999 and 2011. All the children had a significant ASD, diagnosed by Transthoracic Echocardiography (TTE), susceptible to be closed by catheterization according to physicians’ opinions. A significant ASD was defined by a pulmonary-to-systemic flow ratio of more than 1.5 (estimated by Doppler TTE). Patients underwent a physical examination, chest x-ray, EKG, and TTE echo the day before percutaneous closure. A blood test including the usual coagulation tests was performed to exclude thrombopenia or coagulation disorders. The procedures were performed under general anesthesia. An initial Transesophageal Echocardiography (TEE) specified the localization, size, and rims of the ASD.
Parenteral antibiotics were given (cefazolin 30mg/Kg) and vascular access was usually obtained via the femoral vein. Heparin (100IU/Kg) was administered prior to the procedure. During the procedure, the stretched diameter of the ASD was specified using a PTS® sizing balloon (B. Braun Interventional System, Bethlehem, Pennsylvania, USA) measured by angiography and TEE. An exchange guide was placed into the left superior pulmonary vein. Along this guide, a sheath was introduced into the left atrium. The device was deployed under fluoroscopic and echo graphic guidance. Before the release of the device, a Minnesota wiggle (push-pull maneuver) was performed to ensure the correct positioning and stability of the device, and TEE was then performed to rule out any interference from adjacent structures. After the effective release, a last TEE controlled the position of the device, a potential residual shunt, or any interferences with adjacent structures. Patients remained hospitalized for one night under EKG monitoring. Before discharge, every patient underwent an EKG, TTE, chest x-ray, and femoral vessel echography. A 6-month course of aspirin (3-7mg/Kg/d) was initiated, with antibiotic prophylaxis recommended for six months following ASD device placement. Of the 220 patients, 15 patients (6.8%) had an ASD that was too large for device closure and two required surgery after secondary embolization of the device. The 203 children who underwent effective percutaneous closure were included in the follow-up study.
Patient follow-ups were scheduled for Day 7, Day 30, Day 90, Day 180, and Day 360 after ASD closure and consisted of a precise anamnesis, physical examination, EKG, and TTE. Later consultations occurred every year to every 5years, at the cardiologist’s discretion. Patients and their parents were informed and received a brochure containing information on alert symptoms (chest pain, palpitations, or dizziness) requiring rapid check-up with a TTE to rule out pericardial effusion or device migration. The following information was collected from the patients’ medical files: patient’s age, gender, weight and height at catherization, catheterization data (date, procedure time, and fluoroscopy time), and follow-up data (last follow-up date, medical status, complications [embolization, residual shunt, chest pain, palpitations, arrhythmias, neurological indesirable events]). To complete the follow-up, patients who had not been seen at the clinic over the previous year were contacted to collect all missing information. The methods and study protocol were approved by the local ethics committee. Data were expressed in mean±standard deviation.
Results
At catheterization, the mean age was 7.96±4.2 years (6 months to 18 years old), mean weight 29.05±17Kg) (5.1 to 94.5Kg), mean height 124.7±26.9cm (54cm to 185cm). Male-female ratio was 1:2. All patients had a single centrally located ASD2. ASD was an isolated feature in 181/203 patients (89.2%) while ASD was considered as part of a syndrome in 22/203 patients (10.8 %). While in 7 patients, a precise diagnosis related to morphological features was not possible (microcephaly, palate cleft, facial dysmorphia, heterotaxia, intestinal malrotation…), the diagnosis was confirmed as trisomy 21 (n=5, 2.5%); Di George syndrome (n=3, 1.5%); VACTER syndrome (n=1, 0.5%); trisomy 10 (n=1, 0.5%); Noonan syndrome (n=1, 0.5%); Prader-Willi syndrome (n=1, 0.5%); CHARGE syndrome (n=1,0.5%); Ehlers-Danlos syndrome (n=1, 0.5%), fetal alcohol syndrome (n=1, 0.5%). Pre-catheterization evaluation revealed that 95/203 patients (46.8%) reported symptoms, such as respiratory tract infections (n=31, 15.2%), dyspnea on exertion (n=28, 13.8%), growth restriction (n=15, 7.4%), profuse sudation (n=8, 3.9%), severe fatigue (n=8, 3.9%), palpitations (n=6, 3%), chest pain (n=5, 2.5%), clinical signs of cardiac insufficiency (n=4, 2%). The mean diameter of ASD measured by TEE ranged from 6mm to 38mm (mean 13.1±4.4mm), while the balloon-stretched diameter ranged from 6mm to 33mm (mean 16.0±4.7mm). ASD closure device dimension ranged from 8mm to 35mm (mean 19.2 ± 5.7mm). The ASD closure devices used were the AmplatzerTM Septal Occluder (Abbott (Saint-Jude Medical), Saint-Paul, Minnesota, USA) in 151/203 patients (74.4%) and the Helex® Septal Occluders (Gore, Newark, Delaware, USA) in 30/203 patients (14.8%). Other devices were the Cardia® Atria devices (Cardia, Eagan, Minnesota, USA) in 10/203 patients (4.9%), CardioSEAL® Septal Occluder (NMT Medical, Boston, Massachusetts, USA) in 9/203 patients (4.4%) and Occlutech® devices (Occlutech, Helsingborg, Sweden) in 3/203 patients (1.5%).Fluoroscopy time varied between 2min and 80min (mean 17.39±13.76 min). Residual shunts on TEE were observed in 50/203 patients (24.4%) immediately after closure. A residual shunt was observed in 12/203 patients (5.9%) 6 months after the procedure, but none with a pulmonary-to-systemic flow ratio of more than 1.5 by Doppler echo. Major short-term complications were observed in 4/205 patients (Figure 1). Three patients experienced an embolization of the device. One embolization occurred during the procedure. The device was immediately retrieved and a larger one inserted. The other two embolizations occurred during the first 12 hours after catheterization and required surgery. One patient had a significant pericardial effusion 12 hours after the procedure, requiring pericardial drainage with the drain left in place, along with monitoring in ICU for 24 hours with a good evolution.
Minor procedural complications in 12/205 patients (5.8%) were mostly transient arrhythmias or EKG anomalies: three (1.5%) sinus arrhythmias, three (1.5%) atrial premature beats, one (0.5%) ventricular premature beat, one (0.5%) flutter treated successfully with beta-blockade and amiodarone, one (0.5%) auricular tachycardia of spontaneous resolution, one (0.5%) transient Mobitz 1 A-V Block in a patient with a previous episode of first degree A-V Block, one (0.5%) transient junctional rhythm, as well as one (0.5%) transient repolarization abnormalities. Vascular complications were observed in 7/205 patients (3.5%): three (1.5%) subcutaneous hematomas at puncture site, two (1%) mild hemorrhages at puncture site, one (0.5%) arteriovenous fistulas requiring surgery, and one (0.5%) superficial femoral vein thrombosis successfully treated by Low Molecular Weight Heparin (LMWH). Other minor short-term complications were nausea and vomiting in four patients (2%), headaches in three (1.5%), fever in three (1.5%), skin rashes in two (1%), chest pain in one (0.5%), and epigastralgia in another one (0.5%). One patient developed a skin infection at the puncture site, requiring disinfection and antibiotics. In the medium- and long-term, no major complications (death, severe arrhythmia, stroke, or massive bleeding) were reported. In the first month after ASD closure, 17/203 patients (8.4%) experienced minor complications, with 9/203 patients (4.4%) reporting chest pain, and three complaining of headaches, dizziness, and nausea. In 3/203 patients (1.5%), echocardiography revealed very mild pericardial effusion that did not require treatment; one patient (0.5%) was treated with beta-blockers by a general practitioner for palpitations without any work-up prior to treatment; one (0.5%) patient exhibited fever; one patient (0.5%) complained of profuse sudation; one patient (0.5%) complained of short epistaxis. Follow-up and work-up were reassuring, with favorable evolution in all cases.
Seven patients from abroad were lost for follow-up; 20 patients were seen for follow-up only at 1 month (n=6), 6 months (n=9), and 1 year (n=4) after the procedure, due to parents’ “noncompliance” with the planned follow-up schedule. Long-term mean follow-up was 12.9±3 years (0.1–18.3 years), with a total of 1710 patients-year of follow-up. The total elapsed time since ASD closure was 2527 patients-year. One death, recorded several years after catheterization and not related to ASD closure, was reported in a patient with encephalopathy. Some symptoms occurred in 27/176 patients (15%): 12 (6.8%) reported palpitations; five (2.8%) complained of chest pain; nine (5%) described mild dyspnea; four (2.2%) reported headaches. The symptoms were transient and the work-up, when realized, was reassuring in all cases.
Discussion
Since ASD transcatheter closure became an alternative to surgical procedure using cardiopulmonary bypass, closure of the secundum ASD has increasingly switched from a surgical approach to a percutaneous transcatheter approach in most instances. There are only few long-term studies on the efficacy and safety of percutaneous ASD closure in children. The data on all 220 children who were admitted to our catheterization laboratory between 1999 and 2011 for attempted ASD closure were retrospectively reviewed, in conjunction with a study performed at our institution to assess the results of surgical closure during the same study period [4].
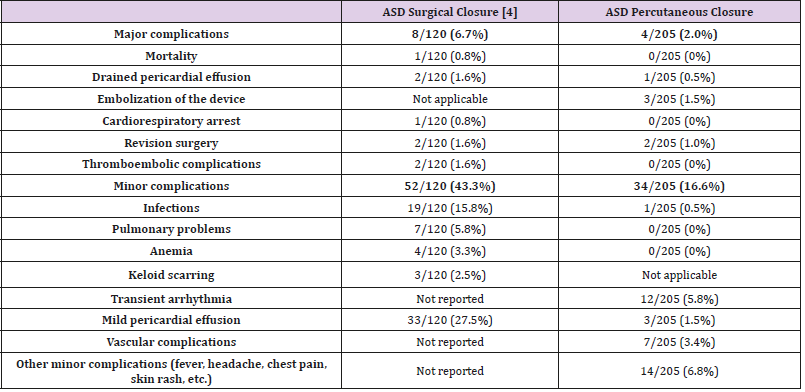
The current study shows that percutaneous ASD closure was feasible in 205/220 children referred to the catheterization lab for ASD closure (93%). The procedure was successful in 203/205 children (99%), with secondary embolization of the device in two cases; very effective with no residual shunt in 191/203 patients (94%); and with a non-significant shunt (QP/QS ratio <1.5) on echocardiography in the 12 patients with a residual shunt. A similar 94% success rate, with no residual shunt at late follow-up, was reported in the literature [5]. Major complications occurred in three patients (1.5%) during the first 24 hours after ASD closure. Surgery was required in two patients, secondary to device embolization (1%), an incidence similar to that reported by another study [6], and one patient required the drainage of a pericardial effusion. We did not experience any fatal issues relating to the procedure nor delayed wall erosion, the latter being a rare complication with a reported incidence of 0.1–0.3% [7-12]. Cardiac perforation and erosion are rare but potentially fatal. Most cases reported in the literature were clustered within the first 6 months after device closure (76%), though erosions were still reported as late as 3 years after deployment [12], with a mortality rate of 0.05%. This observed mortality is still lower than the overall surgical mortality of 0.13% determined from the Society of Thoracic Surgeons database [13]. Other major complications described in the literature were not observed in our series: retroperitoneal hematoma, air embolism [14], deep vein thrombosis, arteriovenous fistula [15], transient ischemic attack [9], late device thrombus formation [6], and late device embolization [16]. These complications, though rare and usually observed during the first days after percutaneous closure, emphasize the need for careful monitoring and follow-up after device closure, in addition to providing appropriate information for the patients and their families on alerting symptoms. Identification of high-risk cases, early recognition, and prompt intervention can minimize the risk of undesirable events [17,8]. Following ASD closure, the incidence of symptoms reported by the patients decreased from 46.7% to 13.3% at late follow-up. Palpitations (12/203) and headaches (4/203), the most common complains, were transient, with reassuring work-up in all cases. Palpitations following ASD closure, as previously reported in the literature [5], were rarely associated with arrhythmia. In comparison with surgical ASD closure, conducted during the same period of time at our institution [4], the results after device closure compare favorably in terms of mortality and morbidity (Table 1). Of note, both studies are retrospective in nature, and the populations were not similar, with patients being younger and exhibiting larger ASD in the surgical group. Therefore, the results cannot be properly compared. Nevertheless, the collected data confirm the good results and relatively low risk of performing a transcatheter percutaneous closure of the secundum ASD. These findings are in line with the results of two multicenter non-randomized trials, confirming a significantly lower complication rate of 7.2% after device closure using the Amplatzer septal occluder versus 24% for the surgical group in the paper published by Du et al. [18], along with an undesirable effect rate of 5.9% for the device group using the Helex septal occluder versus 10.9% for the surgical group in the paper published by Jones et al. [19]. There were no deaths in the device group, whereas there was one death from pericardial tamponade in the surgical group [19]. Due to the ease of implantation and low rate of major complications, percutaneous closure of ASD when technically possible, is currently the preferred therapeutic option. Pre-intervention TTE is essential to delineate the septal defect, evaluate the rims, and detect additional defects or other cardiac abnormalities. Pre-intervention TEE is useful to further specify the septal defect, confirm the size of the rims, specify the relationship between the device and cardiac structures, and control for any complication, both during and at the end of the procedure. As it remains difficult to correctly identify ASD and its rims by TTE, TEE is of particular relevance. Selection of the correct device size, aseptic technique, correct heparinization, stability maneuver, antibioprophylaxis, as well as antiplatelet therapy are likely to decrease the complication rate. In this study, although some historical ASD occluders were employed, the majority of ASD closures were performed using Amplatzer Septal Occluders and, more recently, by means of Occlutech® devices. Actual ASD closure devices are very safe and able to close the vast majority of secundum ASDs. Device closure of very large defects, ASD with small rims, and multifenestrated ASD is still challenging, but can be facilitated by the greater device flexibility following deployment achieved through a bioptome-like delivery system [20]. To decrease exposure to radiation, increased use of TEE is being proposed, as it may be associated with better ASD management, especially in children, who are very susceptible to radiation.
Table 1: Short and medium-term complications after ASD surgical closure [4] versus ASD percutaneous closure.
Study Limitations
During the study period (11 years), ASD closure devices have improved to some extent. The great variety of devices used could be seen a bias for the correct interpretation of our results, given that the numbers for each device were too small to obtain relevant statistics. Retrospective studies inevitably involve selection bias and the lack of some informative parameters.
Conclusion
Percutaneous ASD closure is a safe procedure in children, with regards to medium- and long-term follow-up. This study confirms that percutaneous ASD closure is an appropriate alternative to surgical closure. Despite major advances made in the field of ASD closure devices, along with the increasing experience in implanting such devices, complications tend to persist and cannot be eliminated. Careful patient selection, good understanding of potential complications, appropriate information provided to the patient’s family, and close monitoring after percutaneous ASD closure are all mandatory measures to decrease complications [17-20].
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- Van der Linde D, Konings EE, Slager MA, Witsenburg M, Helbing WA, et al. (2011) Birth prevalence of congenital heart disease worldwide: a systematic review and meta-analysis. Journal of the American College of Cardiology 58(21): 2241-2247.
- Schwedler G, Lindinger A, Lange PE, Sax U, Olchvary J, et al. (2011) Frequency and spectrum of congenital heart defects among live births in Germany: a study of the Competence Network for Congenital Heart Defects. Clinical research in cardiology: official journal of the German Cardiac Society 100(12): 1111-1117.
- King TD, Thompson SL, Steiner C, Mills NL (1976) Secundum atrial septal defect. Nonoperative closure during cardiac catheterization. Jama 235(23): 2506-2509.
- De Beco G, Mambour N, Vo C, Vanhoutte L, Moniotte S, et al. (2018) Recent Experience and Follow-Up After Surgical Closure of Secundum Atrial Septal Defect in 120 Children. Pediatric cardiology 39(7): 1440-1444.
- Knepp MD, Rocchini AP, Lloyd TR, Aiyagari RM (2010) Long-term follow up of secundum atrial septal defect closure with the amplatzer septal occluder. Congenital heart disease 5(1): 32-37.
- Chessa M, Carminati M, Butera G, Bini RM, Drago M, et al. (2002) Early and late complications associated with transcatheter occlusion of secundum atrial septal defect. Journal of the American College of Cardiology 39(6): 1061-1065.
- Crawford GB, Brindis RG, Krucoff MW, Mansalis BP, Carroll JD (2012) Percutaneous atrial septal occluder devices and cardiac erosion: a review of the literature. Catheterization and cardiovascular interventions : official journal of the Society for Cardiac Angiography & Interventions 80(2): 157-167.
- Amin Z, Hijazi ZM, Bass JL, Cheatham JP, Hellenbrand WE, et al. (2004) Erosion of Amplatzer septal occluder device after closure of secundum atrial septal defects: review of registry of complications and recommendations to minimize future risk. Catheterization and cardiovascular interventions: official journal of the Society for Cardiac Angiography & Interventions 63(4): 496-502.
- Chan KC, Godman MJ, Walsh K, Wilson N, Redington A (1999) Transcatheter closure of atrial septal defect and interatrial communications with a new self-expanding nitinol double disc device (Amplatzer septal occluder): multicentre UK experience. Heart (British Cardiac Society) 82(3): 300-306.
- McElhinney DB, Quartermain MD, Kenny D, Alboliras E, Amin Z (2016) Relative Risk Factors for Cardiac Erosion Following Transcatheter Closure of Atrial Septal Defects: A Case-Control Study. Circulation 133(18): 1738-1746.
- Diab K, Kenny D, Hijazi ZM (2012) Erosions, erosions, and erosions! Device closure of atrial septal defects: how safe is safe? Catheterization and cardiovascular interventions : official journal of the Society for Cardiac Angiography & Interventions 80(2): 168-174.
- Preventza O, Sampath-Kumar S, Wasnick J, Gold JP (2004) Late cardiac perforation following transcatheter atrial septal defect closure. The Annals of thoracic surgery 77(4): 1435-1437.
- Di Bardino DJ, Mc Elhinney DB, Kaza AK, Mayer JE (2009) Analysis of the US Food and Drug Administration Manufacturer and User Facility Device Experience database for adverse events involving Amplatzer septal occluder devices and comparison with the Society of Thoracic Surgery congenital cardiac surgery database. The Journal of thoracic and cardiovascular surgery 137(6): 1334-1341.
- Fischer G, Stieh J, Uebing A, Hoffmann U, Morf G, et al. (2003) Experience with transcatheter closure of secundum atrial septal defects using the Amplatzer septal occluder: a single centre study in 236 consecutive patients. Heart (British Cardiac Society) 89(2): 199-204.
- Wagdi P (2011) Closure of Interatrial Septal Communications: Adverse Events and Lessons Learned. Cardiology research 2 (1): 7-15.
- Wagdi P, Kunz M (2008) Percutaneous, minimally invasive retrieval of a dislodged and impacted device from the abdominal aorta, four months after closure of an atrial septal defect. The American journal of cardiology 102(8): 1111-1112.
- Jenkins K, Ringel R, Rome J, Vincent R, Martin G, et al. (2013) Transcatheter device closure of atrial septal defects: a safety review. JACC Cardiovascular interventions 6(5): 433-442.
- Du ZD, Hijazi ZM, Kleinman CS, Silverman NH, Larntz K (2002) Comparison between transcatheter and surgical closure of secundum atrial septal defect in children and adults: results of a multicenter nonrandomized trial. Journal of the American College of Cardiology 39(11): 1836-1844.
- Jones TK, Latson LA, Zahn E, Fleishman CE, Jacobson J, et al. (2007) Results of the U.S. multicenter pivotal study of the HELEX septal occluder for percutaneous closure of secundum atrial septal defects. Journal of the American College of Cardiology 49(22): 2215-2221.
- Kenny D, Eicken A, Dahnert I, Boudjemline Y, Sievert H, et al. (2019) A randomized, controlled, multi-center trial of the efficacy and safety of the Occlutech Figulla Flex-II Occluder compared to the Amplatzer Septal Occluder for transcatheter closure of secundum atrial septal defects. Catheterization and cardiovascular interventions: official journal of the Society for Cardiac Angiography & Interventions 93(2): 316-321.

 Research Article
Research Article