Abstract
Background: Pulpitis is a dental pulp tissue inflammation usually caused by bacteria invasion that stimulates the release of inflammation mediators. TNF-α is the most common acute inflammation mediator. The increase of TNF-α level causes systemic reaction. Recently, the usage of plants as medication has been widely researched. One of them is Sarang semut (Myrmecodia pendans) that contains flavonoids as antiinflammation. This experimental laboratory study with pre-post treatment and control group design was done to find out the effect of Myrmecodia pendans (M.pendans) ethanol extract towards the blood TNF-α level in Sprague Dawley that has pulpitis by 0,01mL Porphyromonas gingivalis induction for 48 hours.
Methods and Material: The study subject was divided into Group I (negative control), group II (pulpitis group), group III (pulpitis treated with M.pendans), and group IV (pulpitis treated with Ca(OH)2/positive control). Group III and IV divided into subgroups (48, 96, 168, and 336 hours). Blood TNF-α level was measured by ELISA (BioLegend’s Rat TNF-α LEGEND-MAX™).
Result: One way ANOVA showed significant differences (p<0.05) between the pulpitis group treated with Ca (OH)2 and M.pendans groups at day 4 (96 hours), 7 (168 hours), and 14 (336 hours). Post Hoc LSD showed significant differences between pulpitis group with Ca (OH)2 and M.pendans with (p=0.001<0.005) and (p=0.019<0.005) respectively at day 4.
Conclusion: Ethanol extract of M.pendans has anti-inflammation effect that able to decrease blood level of inflammation mediator TNF-α after day 4. Moreover, on day 7 the TNF-α level is lower than those of Ca(OH)2. The anti-inflammation effect of M.pendans equals to those of Ca(OH)2 as a commercial product of inflamed pulp tooth at day 14.
Keywords:: Sarang Semut (Myrmecodia Pendans); Pulpitis; Anti Inflammation; TNF-α; ELISA
Introduction
Inflammation is a normal respond protective of tissue toward physical traumatic, chemical injury or microbiology activity [1]. Inflammation divided into acute and chronic [2]. Pulpitis is inflammation of pulp tissue that commonly occurred and often found on dental practice. Pulpitis may due to variety of factors such as bacterial invasion, trauma and iatrogenic [3,4]. Pulp inflammation activates variety of biological system such non specific inflamed reaction mediated by histamine, bradykinin, and arachidonic acid [5]. The final stage of inflamed respond is to deliver the protein plasm and phagocyte into injured area for isolation, destruction or inactivation of incoming agent, clean the debris and prepare tissue for wound healing process [6]. Porphyromonas gingivalis (P.gingivalis) is an anaerobic Gram negative bacteria that expressed several virulent factors like fimbriae lekin-like adesina, polysaccharide capsule, lipopolysaccharide, hemagglutinin, hemolytic, vesicle membrane, and proteolytic enzymes that cause inflam-mation of gingiva, tissue destruction and tooth loss [7]. Lipopolysaccharide (LPS) is an important part of P.gingivalis that responded by inflammatory cells that release inflammatory mediators indeed inflamed reaction [8]. Tumor Necrosis Factor Alpha (TNF-α) is a derivative protein of monocyte that has pro inflammatory and immunomodulatory effect. This type of cytokine as inflammatory mediator will stimulate bone resorption, prostaglandin synthesis, and protease production by cells such as fibroblast and osteoblast [9]. TNF-α is also a main cytokine of acute inflamed respond towards negative Gram bacteria.
The severed infection may trigger a lot of TNF-α production resulted in systemic reaction. Lipopolysaccharide is a potent stimulant to TNF-α secretion by macrophage [10]. TNF-α is suspected as an important inflammatory mediator. Increase of TNF-α has toxic potential effect like hypersensitivity reaction [11,12]. Indonesia has a lot of potential herbal plants [13]. Medical world recently used traditional medicine through the usage of medicinal plants. One of these potential plants is Sarang semut (Myrmecodia pendans) that used empirically as anti tumor, anti-cancer and diabetes remedies [14]. Ethanol extract of this plant has anti inflammation [15]. The phytochemical content of this plant like flavonoid compound, triterpenoid/steroid and saponin [16,17]. Previous study revealed that the flavonoid content has anti inflammation activity [17,18]. Flavonoid has role in inactivation of carcinogenic and cells proliferation also inhibition of angiogenesis [19]. These activities have been proven by previous studies. However there is no specific study of this fact on hard tissue therefore the purpose of this in vivo study is to find out the anti-inflammation effect of ethanol extract M.pendans toward the blood TNF-α level of Sprague Dawley rats that has molar tooth induced pulpitis by P.gingivalis. The blood TNF-α level was measured by ELISA method.
Methods and Materials
This study was approved by the Animal Care and Use Committee (ACUC) Veterinary Teaching Hospital, Animal Medical Faculty, Bogor Agricultural University (No.352016 ACUC RSHP FKH-IPB).
Subjects
This in vivo pre and post experimental laboratory study with control used subjects of male Sprague Dawley rats with the age of 16-17 weeks and body weight of 150-350gr. The total sample (N=27) [20] were divided in groups and subgroups by simple random sampling (n=3). The inclusion criteria of samples are as followed: pure origin, healthy and active, normal attitude, no physical abnormality. The exclusion criteria of subjects are pathologic condition of their teeth and subjects which died within period of research.
Pulpitis Induction
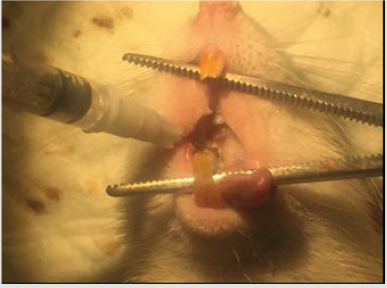
Subjects (N=27) was divided by simple random sampling into group I (negative control group, n=3), group II (pulpitis group, n=3), group III (pulpitis treated with ethanol extract M.pendans,n=9), group IV (pulpitis treated with Ca(OH)2/positive control, n=9). Group III and IV was divided into 3 sub groups: 96 hours, 168, 336 hours with each n=3. As the first step of this research, pulpitis induction was done by applied intraperitoneal anesthesied on subjects of group II, III, and IV used 65mg/kg body weight of Ketamin (Ketalar®, Warner Lambert, Irlandia) and 7mg/kg body weight of xylazine HCl (Rompun®, Bayer, Leverkusen, German). The class I Black cavity was performed on occlusal surface of first upper right molar tooth of subject used 0.01 mm round bur with low speed for the depth as much as 0.01 mm up to the pulp chamber roof. Isolated the working area and drop the 0.01mL suspension of P. gingivalis (3x108/25mL CFU) into the cavity (Figure 1). Covered the cavity for 48 hours with the glass ionomer cement (GIC) as temporary filling (Figure 2).
Figure 1: Pulpitis induction on the class I Black cavity of first upper right molar tooth by 0.01mL P.gingivalis (3x108/25mL CFU).
Pulpitis Treatment
On day 2 (48 hours), the temporary filling of group III and IV was opened. Put the ethanol extract
M.pendans
and Ca(OH)2 on pulpitis group treated withM.pendans
(group III) and positive control (group IV) respectively.Repeated this method on day 4 (48 hours), 7 (168 hours), and 14 (336 hours) both for pulpitis treated with M.pendans and positive control groups. Group II was no treated pulpitis group.
The Blood Sample Collection
Subjects were general anesthetized. The blood samples were collected after the execution of subjects on day 2, 4, 7, 14 for 3 subjects of each group. As much as 4mL heart blood was collected that injected into the tube and centrifuged 1.000rpm for 10 minutes to filtrate the serum. Put the serum into tube and keep on -20 0C before tested with ELISA method for the TNF-α level (Figure 3).
TNF-α Level Measurement
The measurement of TNF-α blood sample from heart of subject was done on Clinical Pathology Laboratory of Dharmais Hospital using sandwich method of ELISA. First, prepare the sample and reagent (Figure 4). The standard concentrations used in this phase are 3.9pg/ml, 7.8pg/ml, 15.6pg/ml, 31.25pg/ml, 62.5pg/ ml, and 125pg/ml. Wash the plate as much as 4 times used wash buffer solution that diluted with aquadest with ratio 1:19 and put the 50μl of matrix A solution into the well to get standard. Then put the 50μl Assay Buffer A solution into well as sample. Put 50μl of diluted standard in to well for standard and add 50μl diluted sample into well for sample and then lock the plate using sealer plate to be incubated for 2 hours with vibration. Wash the plate as much as 4 times used wash buffer and put 100μl antibody detected solution into each well and lock the wells to be incubated for 1 hour with vibration. Wash the plate as much as 4 times used wash buffer, put 100μl Avidin-HRP D solution and lock the plate to be incubated for 30 minutes with vibration (Figure 5). Add 100μl stop solution (Figure 6). The absorbance was measured by λ=450nm within 30 minutes (Figure 7).
Result and Discussion
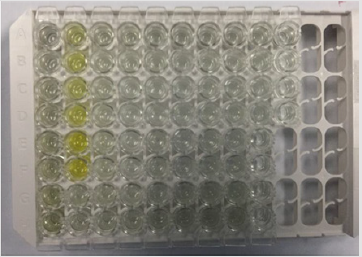
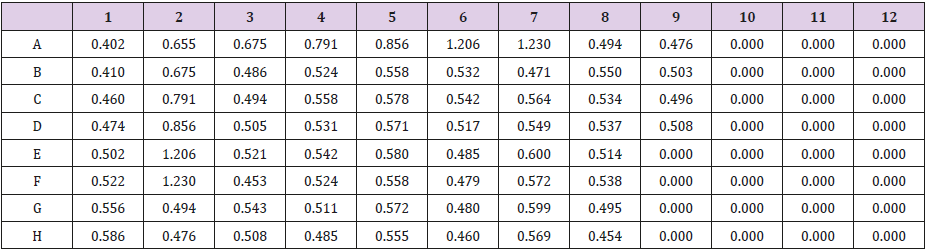
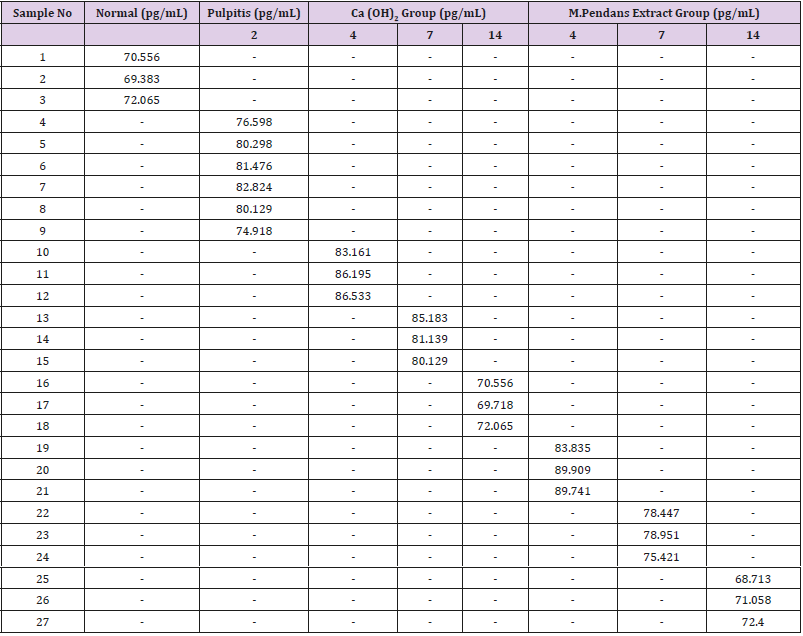
The samples that were placed within well needs to be mapping at the ELISA kit (Table 1). The plate of samples was measured with the ELISA reader (λ=450 nm) within 30 minutes to obtain the absorbance value (Table 2). The results was then calculated with computer-based curve fitting software using a Four Parameter Logistic (4PL) curve fitting algorithm (Figure 8). Fit results was obtained from the software regression formula of four parameter logistic as followed:
Figure 8: Curve-fitting software used regression formula of Four Parameter Logistic.
Note: MSE: The Mean Square Error. (The closer to zero the better fit); R2: This is 1 minus the ratio SS. This will equal 1 for a good fit, and tend towards 0 for a bad fit; SS: The sum of the squares od the residuals. (The closer to zero the better fit); SYX: The standard deviation of the residuals. (The closer to zero the better fit).
Table 1: Well mapping of samples at ELISA reader (λ=450nm).
Note: BL: Blank; ST: Standard; SP: Sample; U: Unused; ST1= 3.9pg/mL ; ST2= 7.8 pg/mL ; ST3= 15.6pg/mL; ST4= 31.25pg/mL; ST5= 62.5pg/mL ; ST6= 125pg/mL ; SP 1-3= negative control/ normal; SP 4-9= induced pulpitis without treatment ; SP 10-12= positive control 96 hrs (H+4) ; SP 13-15= positive control 168 hrs (H+7); SP 16-18= positive control 336 hrs (H+14); SP 19-21= with extract treatment 96 hrs (H+4); SP 22-24= with extract treatment 168 hrs (H+7); SP 25-27= with extract treatment 336 hrs (H+14).
a=0.05009
b=0.9683x
c=236600
d=1128
MSE=0.0004429
R2=0.9932
SS=0.005315
SYX=0.02578
The absorbance value was calculated by curve-fitting software used regression formula of Four Parameter Logistic (4PL)
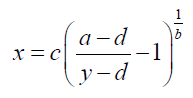
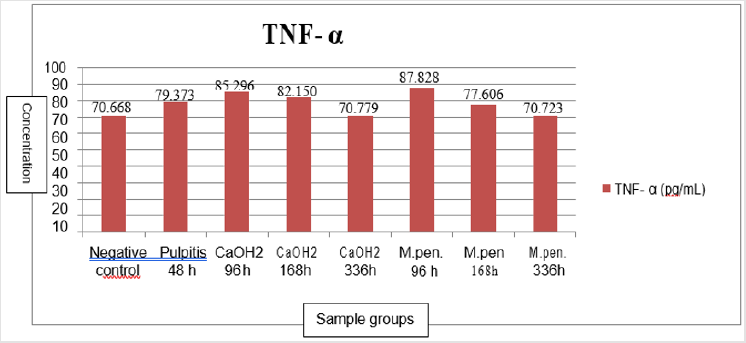
to find out the distribution of TNF-α level on day 2, 4, 7, 14 among subjects (Table 3 & Figure 9): From Table 3, it was shown that the TNF-α blood level increase within the pulpitis group, both Ca(OH)2 and M.pendans groups at day 4 and 7. At day 14 the TNF-α blood level starting to decrease especially on M.pendans group. Figure 9 showed the mean score of TNF-α among groups of sample. It was shown that the level starting decrease on day 14 (336 hours). Shapiro-Wilk normality test showed the normal distribution of data with p>0.05. The homogen test showed the homogenous of data with p>0.05. One way ANOVA test showed the significant differences (p<0.05) among negative control, pulpitis, pulpitis treated by Ca(OH)2 or positive control, and pulpitis treated by M.pendans on day 4 (p=0.000<0.05), day 7 (p=0.000<0.05), and day 14 (p=0.002<0.05). Post Hoc LSD test showed significant difference on day 4 (96 hours) between negative control, pulpitis, and pulpitis treated by Ca (OH)2, and pulpitis treated by M.pendans. There was also significance difference between pulpitis with pulpitis treated by Ca (OH)2 and pulpitis treated by M.pendans; between pulpitis treated by Ca(OH)2 and M.pendans. On day 7 (168 hours) there was significance difference between negative control with pulpitis, pulpitis treated by Ca (OH)2 and M.pendans; between pulpitis treated by Ca(OH)2 and M.pendans. However there was no significant difference between pulpitis treated by Ca (OH)2 and M.pendans. On day 14 (336 hours) there was significance difference between negative control, pulpitis, pulpitis treated by Ca (OH)2 and M.pendans. However there was no significant difference between pulpitis treated by Ca (OH)2 and M.pendans.
Discussion
Inflammation on pulp tissue activates several biological system responds. Mast cell within inflamed pulp tissue produces histamine, leukotriene, and platelet activating factor. Injury of mast cell triggers release of histamine and bioactive substance on granule of cell [21]. One of the most cytokine found within acute inflammation is tumor necrosis factor alpha (TNF-α) which is produced by mast cells, active macrophage, endothelial cell. This type of cytokine is the main type of cytokine on inflamed respond toward negative Gram bacteria. The secretion of TNF-α that stimulated by microbial product likes endotoxin, immune complex and T lymphocyte, especially the lipopolysaccharide as potential stimulus for macrophage releases the TNF-α. TNF-α stimulates adhesion molecule expression on endothelial cell resulted in the increase of adhesion toward leukocyte and cytokine production [22,23]. The high increase of TNF-α insult the systemic reaction [24]. In inflammation, TNF-α has role to increase the prothrombotic function, stimulate adhesion molecule of leukocyte, induct the endothelial cell, manage macrophage activity and immune responding within tissue by stimulate growth factor and other cytokines [24,25]. Today, medicinal plant widely used such as Myrmecodia pendans that has phytochemical compounds such as flavonoid, triterpenoid/steroid, and saponin [14,16,17]. Previous study revealed that flavonoid has anti inflammation, antioxidant, and antimicrobial, defend the neutrophil degranulation, leukocyte accumulation, inflammatory mediator releasing like histamine and prostaglandin [26,27].
Post Hoc LSD test showed that on day 4, there was significant difference between pulpitis treated by M.pendans and positive control/Ca(OH)2 group whereas the TNF-α was higher within M.pendans compared to those on Ca(OH)2 group that means the anti-inflammation effect of M.pendans on day 4 is lower than those of Ca(OH)2 as control positive. However on day 7 and 14 showed that the anti-inflammation effect of M.pendans is higher to the control positive Ca(OH)2 as shown on Figure 9. Figure 9 showed that the TNF-α level increase on pulpitis group day 2 and day 4 both on M.pendans and Ca(OH)2 group. It means that acute inflammation process started from day 1 up to day 4. After that, on day 7 the TNF-α level decrease both on pulpitis treated by M.pendans and Ca(OH)2. It means that the inflammation phase of wound healing process has been decreased to go to later stage of healing process [2].
Conclusion
Ethanol extract of M.pendans has anti-inflammation effect that has been proven by the decrease of inflammation mediator TNF-α blood level after day 4. On day 7, the TNF-α blood level of Ca (OH)2 group is lower than M.pendans groups. However, on day 14, the anti-inflammation effect of M.pendans equals to those of Ca(OH)2 as a commercial product of inflamed pulp tooth.
References
- Dewi TS, Puspawati NM, Suarya P (2015) Aktivitas Antiinflamasi Ekstrak Eter Kulit BatangTenggulun (Protiumjavanicum Burm) Terhadap Edema Pada Tikus Wistar Yang Diinduksi Dengan Karagenan. Journal of Chemistry 9(1).
- Mitchell RN, Cotran RS (2007) Buku Ajar Patologi. Edisi 7 Jakarta, EGC, Indonesia, p. 35-36.
- Iqbal M, Kim S, Yoon S (2007) An Investigation into Differential Diagnosis of Pulp and periapical Pain: A Pennendo Database Study. J Endod 33(5): 548-551.
- Garg N, Garg A (2014) Textbook of Endodontics. (3rd Edn), Jaypee Brothers Medical Publisher, India, p. 22-30.
- Torabinejad M (1994) Mediators of Acute and Chronic Periradicular Lesions. Oral Surg Oral Med Oral Pathol 78(4): 511-521.
- Corwin EJ (2008) Handbook of Pathophysiology. (3rd Edn), Lippincott Williams & Wilkins, Philadelphia, USA.
- Suwandi T (2010) Perawatan Awal Penutupan Diastema Gigi Goyang Pada Penderita Periodontitis Kronis Dewasa. Jurnal PDGI 59(3): 105-109.
- Newman MG, Takei HH, Klokkevold PR, Carranza FA (2006) Carranza’s Clinical Periodontology (10th Edn) St Louis, Saunders, Elsevier Inc, Missouri, USA.
- Girardin E, Roux Lombard P, Grau GE, Suter P, Gallati H, et al. (1992) Imbalance between Tumour Necrosis Factor-Alpha and Soluble TNF Receptor Concentrations in Severe Meningococcemia. The J5 Study Group. Immunology 76(1): 20-23.
- Baratawidjaya KG, Rengganis I (2010) Imunologi Dasar. Edisi 9 FKUI, Jakarta, Indonesia.
- Morrison DC, Danner RL, Dinarello CA, Munford RS, Natanson C, et al. (1994) Bacterial endotoxins and pathogenesis of Gram-negative infections. Current status and future direction. J Endotoxin Res 1(2): 71-83.
- Levine B, Kalman J, Mayer L, Fillit HM, Packer M (1990) Elevated circulating levels of tumor necrosis factor in severe chronic heart failure. N Engl J Med 323(4): 236-314.
- Fatmawati D, Puspitasari PK, Iwang Y (2011) Efek Sitotoksik Ekstrak Etano Sarang Semut (Myrmecodia pendens) pada Sel Line Kanker Serviks HeLa: Uji Eksperimental Secara In Vitro. Sains Medika 3(2): 117-120.
- Takukude P, LohoL, Lintong P (2014) Gambaran Histopatologi Hati Mencit Swiss yang Diberi Air Rebusan Sarang Semut (Myrmecodia pendans) Paska Induksi Dengan Carbon Tetrachlorida (CCl4). EBiomedik 2(2): 10-16.
- Djafar W (2010) Uji Efek Antiinflamasi Umbi Sarang Semut Pada Mencit (Mus musculus) Jantan. Skripsi. Makassar, Indonesia.
- Dirgantara S, Nawawi A, Insanu M (2013) Uji Aktivitas Antioksidan Tiga Spesies Tanaman Sarang Semut (Famili: Rubiaceae) Asal Kabupaten Merauke, Papua. Jurnal Biologi Papua 5(1): 10-16.
- Sudiono J, Tri Oka C, Trisfilha P (2015) The Scientific Base of Myrmecodia pendans as Herbal Remedies. British Journal of Medicine & Medical Research 8(3): 230-237.
- Subroto A, Saputro H (2007) Gempur Penyakit dengan Sarang Semut. PT Gramedia Pustaka, Indonesia.
- Ren W, Qiao Z, Wang H, Zhu L, Zhang L (2003) Flavonoids: Promising Anticancer Agents. Medicinal Research Reviews 23(4): 519-534.
- Federer WT (2005) Experimental Design: Theory and Application (Rancangan Percobaan Teori Aplikasi). Raja Grafindo Persada, Indonesia.
- Torabinejad M, Walton RE, Holland GR (2009) Endodontics: Principles and Practice (4th Edn), Elsevier Health Science.
- Aster JC, Abbas AK, Robbins SL, Kumar V (2013) Robbins Basic Pathology (9th Edn), Elsevier Saunders, Philadelphia, USA.
- Walsh LJ, TrinchieriG, Waldorf HA, Whitaker D, Murphy GF (1991) Human dermal mast cells contain and release tumor necrosis factor alpha, which induces endothelial leukocyte adhesion molecule 1. Proc Natl Acad Sci 88(10): 4220-4224.
- Levine B, Kalman J, Mayer L, Fillit HM, Packer M (1990) Elevated circulating levels of tumor ecrosis factor in severe chronic heart failure. N Engl J Med 323(4): 236-241.
- Soeroso A (2007) Sitokin. Jurnal Oftalmologi 5: 171-180.
- Ilavarasan R, Mallika M, Venkataraman S (2005) Anti-inflammatory and Antioxidant Activities of Cassia fistula Bark Extracts. Afr J Traditional CAM 2(1): 75-80.
- Lakhanpal P, Rai DK (2007) Quercetin: a Versatile Flavonoid. Journal of Medical Update 2(2).

 Research Article
Research Article