Abstract
Background: Acute pancreatitis (AP), often presenting with systemic inflammatory response syndrome (SIRS), is a common gastroenterological condition requiring emergent intensive care. Severe cases of AP with SIRS and associated organ failure have high morbidity and mortality. Early, aggressive hydration is the recommended treatment. We investigated the influence of fluid resuscitation on organ failure, inhospital mortality and 30-day mortality in patients with acute pancreatitis and SIRS.
Methods: Data of 317 patients from the MIMIC III 2002-2012 database who were diagnosed with AP, aged >20 years and had an ICU stay >48 hours were included in the analytic sample. Fluid input, urinary output and cumulative fluid resuscitation were measured at 12-hour intervals. Demographics (age, gender, race/ethnicity) and SIRS clinical manifestations (pulse, respiration, temperature and WBC) were analyzed. Endpoints were organ failure, in-hospital mortality and 30-day mortality.
Results: Odds of organ failure increased significantly with increased fluid input at 0-12 hours and 36-48 hours (adjusted odds ratio [aOR] = 1.125, 95% confidence interval [CI] = 1.019-1.243; aOR = 1.207, 95% CI = 1.0002-1.457), and with increased fluid balance at 0-12 hours and 36-48 hours (aOR = 1.103, 95% CI = 1.004-1.210; aOR = 1.180, 95% CI = 1.009-1.380, respectively). Odds of in-hospital mortality increased significantly with increased fluid input at 24-36 hours (aOR = 1.205, 95% CI = 1.058- 1.373) and increased fluid balance at 24-36 hours (aOR = 1.196, 95% CI = 1.049-1.364). Odds of 30-day mortality increased significantly with increased fluid input at 24-36 hours (aOR = 1.175, 95% CI = 1.028-1.343) and increased fluid balance at 24-36 hours (aOR = 1.188, 95% CI = 1.038-1.359).
Conclusion: In fluid resuscitation for ICU patients with AP and SIRS, amounts of early fluid input (first 12 hours) or cumulative fluid resuscitation within 48 hours are associated with organ failure. Amounts of fluid input from 24 to 36 hours or cumulative resuscitation after 36-48 hours are associated with in-hospital mortality, and fluid balance has similar effects. Fluid output has no association with organ failure or mortality in this patient population.
Keywords: Acute pancreatitis; Intensive care; Fluid resuscitation; Organ failure; Systemic inflammatory response syndrome
Abbreviations: AP: Acute Pancreatitis; DM: Diabetes Mellitus; ICU: Intensive Care Unit; MIMIC: Multi-Parameter Intelligent Monitoring in Intensive Care; SIRS: Systemic Inflammatory Response Syndrome; CI: Confidence Interval; OR: Odds Ratio; aOR: Adjusted Odds Ratio
Introduction
Acute pancreatitis (AP), or inflammation of the pancreas, is a common gastrointestinal condition sometimes associated with systemic inflammatory response syndrome (SIRS), and usually requiring emergent hospitalization [1]. The international classification system introduced by Dellinger et al. [2] describes acuity ranging from mild acute pancreatitis to severe to critical acute pancreatitis characterized by the presence or absence of local (pancreatic necrosis) and systemic (organ failure) markers of severity. Severe and critical AP are common causes of ICU admissions [3], and have high morbidity and mortality [4,5]. The incidence of AP in Europe is 100 in 100,000 population [6], and over 270,000 ICU admissions in the United States are attributed to AP annually, while global incidence of AP continues to rise [7]. Diagnosis of AP is made based on sudden-onset abdominal pain that may radiate to the back and vomiting, with confirmation provided by markedly elevated (three times normal) levels of serum amylase or lipase [1,8].
Enhanced computed tomography may be performed if
biochemical tests are inconclusive, or if indications of local
complications or another acute abdominal condition such as
gastrointestinal tract perforation are noted [9]. Many ICU admissions
for acute pancreatitis and SIRS are associated with significant
morbidity in the early stages, which may lead to prolonged
hospitalization or death, particularly when other organ systems are
affected [3,10]. Guidelines of the 2012 International Association
of Pancreatology/American Pancreatic Association (IAP/APA)
[8] and the American College of Gastroenterology [9] advise that
aggressive hydration should be provided to all patients with organ
failure and/or SIRS except when precluded by cardiovascular and/
or renal comorbidities. Early aggressive intravenous hydration with
Ringer’s lactate is reported to be most beneficial within the first 12-
24 hours [1,7], serving to restore fluid balance (circulating volume)
and output, although further benefits may not be realized [9].
Studies have shown that fewer patients receiving fluid
resuscitation developed SIRS and that C-reactive protein (CRP)
levels were also lower, indicating reduced inflammation, but patient
outcomes remained similar [10,11]. Fluid therapy is a complex
process with noted risks due to possible volume overload, and
continuous monitoring is essential to assess patient response [3].
Because excessive fluid administration may lead to organ failure,
abdominal compartment syndrome, sepsis, need for intubation,
and in-hospital mortality, the type, rate, and amount of fluids is
critical during the initial 12-24 hours in ICU and throughout 72-
hour cumulative fluid resuscitation [12,13]. Mole et al. [14] suggest
that fluid prescriptions must be individualized based on patients’
specific physiological derangement. Other authors suggest that
optimization of fluid resuscitation in treating AP needs further
study and consensus [13,15]. However, consensus is still lacking
on specific types of fluid therapy, administration rates, amounts of
fluid used, and desired end points [16].
The Multi-parameter Intelligent Monitoring in Intensive
Care (MIMIC) III database is maintained by the Laboratory
for Computational Physiology at the Massachusetts Institute
of Technology (Cambridge, MA, USA). The MIMIC III database
encompasses a diverse and exceptionally large population of ICU
patients and has been used to support a diverse range of analytic
studies [17]. It is freely accessible to researchers and we determined
that it would provide an ideal data source for the present study.
Our hypothesis was that fluid resuscitation used to resolve the
complex clinical dilemma of AP, with or without SIRS, was likely
associated with organ failure and mortality. Therefore, this study
aimed to examine the influence of fluid resuscitation (fluid input,
fluid output, and fluid balance) on the occurrence of organ failure,
in-hospital mortality and 30-day mortality in patients with acute
pancreatitis with or without SIRS.
Methods
Data Source
The present study obtained all patient data from the Medical Information Mart for Intensive Care III (MIMIC-III ) 2002-2012. MIMIC- III is a large, freely accessible database that includes de-identified health-related data collected from over 40,000 patients admitted to critical care units of Beth Israel Deaconess Medical Center (BIDMC), Boston, MA, USA, between 2001 and May 2012. The MIMIC database website (https://mimic.physionet.org/about/ mimic/) states that the BIDMC Department of Medicine is supported by grants (R01-EB001659, 2003-2013; R01-EB0117205, 2014- 2018) from the National Institute of Biomedical Imaging and Bioengineering (NIBIB) of the National Institutes of Health (NIH). This research database is a joint venture managed by researchers from the Laboratory for Computational Physiology at Massachusetts Institute of Technology (MIT). Use of the MIMIC is approved by the Institutional Review Boards of BIDMC and MIT and use of the database is granted to investigators for research purposes.
Study Population
We screened the MIMIC III database of 46,520 ICU patients for patients older than 20 years who were diagnosed with acute pancreatitis and had stayed in ICU more than 48 hours. For patients with more than one ICU stay, only the first ICU stay was eligible for inclusion. Patients who were pregnant, diagnosed with renal failure or had undergone dialysis, had non-systemic inflammatory response syndrome and were diagnosed with heart failure, diabetes mellitus (DM), or pancreatic cancer were excluded. Finally, the data of 317 ICU patients who met the inclusion criteria were included for analysis.
Study Design
We conducted a retrospective cohort study to evaluate the associations between fluid resuscitation performed within 24 to 48 hours of ICU admission, and the occurrence of SIRS and patients’ length of stay in ICU. The study endpoints were organ failure, inhospital mortality and 30-day mortality. Demographic data included in the analysis were subjects’ age, gender and race/ethnicity (categorized by MIMIC as white, black and others). Variables of SIRS clinical manifestations included for analysis were pulse>90 beats per minute, respirations >20 per minute, temperature <96.8oF or >100.4oF, and white blood cell (WBC) count>12000 cells/ mm3.
Fluid Resuscitation
The volume and type of fluid input and output were recorded at 12, 24, 36, and 48 hours after ICU admission. Fluid balance (FB) was calculated as fluid intake minus fluid output and dichotomized into two groups based on the median. Percentages of colloids, nutrition, hypertonic crystalloid and non-hypertonic crystalloids in total fluid input were used to define types of fluid input. All evaluations were performed to compare the effects of volume and types of fluid resuscitation within 12, 24, 36 and 48 hours.
Statistical Analysis
Continuous variables are expressed as the mean ± standard deviation (SD) and median value (interquartile range, IQR). Categorical variables are expressed as counts (percentage). Univariate and multivariate logistic regression analyses were performed to examine the impact of fluid resuscitation on organ failure, in-hospital mortality and 30-day mortality. The multivariate regression model of organ failure was adjusted simultaneously for age, gender, race, heart rate, and WBC. The multivariate regression model of in-hospital mortality and 30-day mortality was adjusted simultaneously for age, gender, race, temperature, WBC, and organ failure. All statistical assessments were two-sided and evaluated at the 0.05 level of significance. Statistical analyses were performed by IBM SPSS statistical software version 22 for Windows (IBM Corp., Armonk, New York, USA).
Results
A total of 46,520 inpatients were recruited from the Medical Information Mart for Intensive Care III (MIMIC-III) database from which the data of 951 inpatients aged ≥ 20 years old and diagnosed with acute pancreatitis were considered eligible for inclusion in the present study. After excluding 288 patients with an ICU stay of less than 48 hours, 6 patients who were missing ICU measurement information, 1 pregnant woman, 11 patients who were on dialysis, 186 patients with heart failure, 134 patients with DM, 1 patient with pancreatic cancer and 7 patients with non-systemic inflammatory response syndrome, the data of a total of 317 patients were included in the analytic sample.
Subjects Demographic and Clinical Characteristics
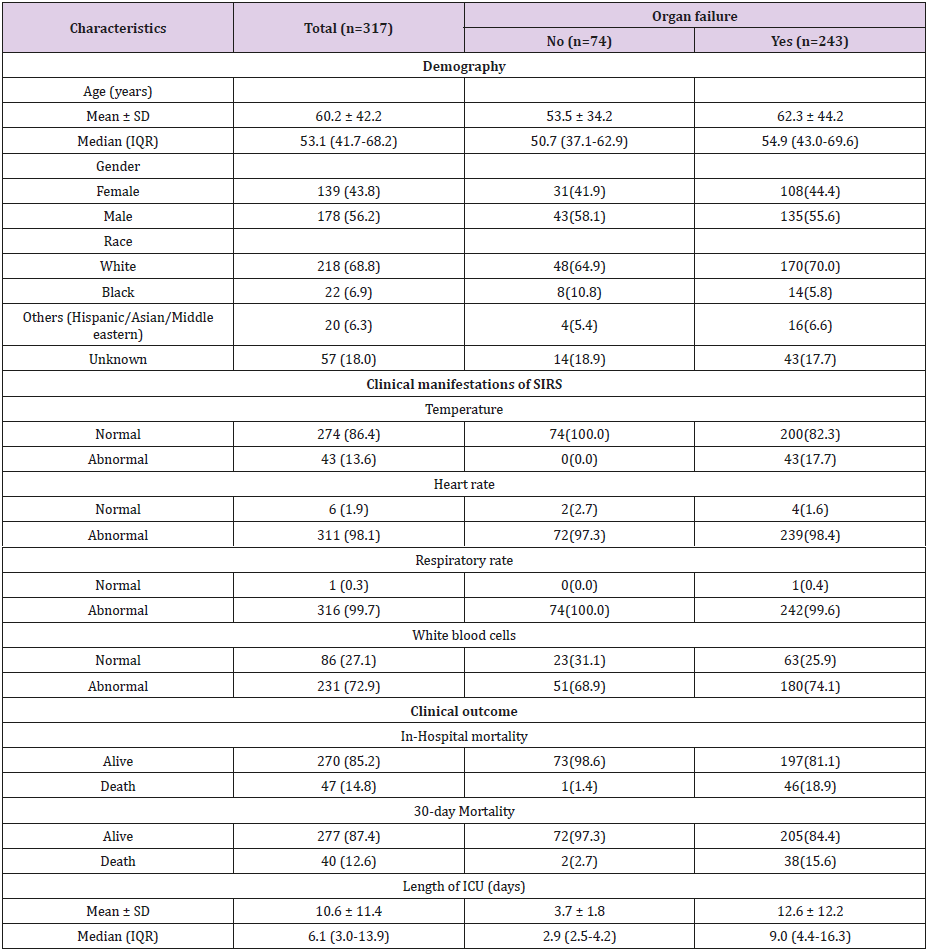
Subjects’ characteristics are summarized in Table 1. The mean age of the study population was 60.2 years, made up of 139 females (43.8%) and 178 males (56.2%), in which 74 patients without organ failure (23.3%) and 243 patients with organ failure (76.7%). The mean of length of stay in ICU was 10.6 days. In-hospital mortality of the study population was 14/8% and 30-day mortality was 12.6% (Table 1). At a mean time of 12 hours, the average fluid balance was 3.2 L, with fluid input of 4.6 L and fluid output 1.4 L. Average fluid balance at 24 hours, 36 hours, and 48 hours were 1.9L, 1.6 L, and 1.1L, respectively (Figure 1A). Cumulative fluid balance at 24 hours, 36 hours, and 48 hours were 5.1 L, 6.7 L, and 7.8 L, respectively (Figure 1B).
Table 1: Subjects’ Demographic and Clinical Characteristics.
Note: Continuous Variables were shown Mean ± SD and Median (IQR); Categorical Variables were Shown Counts (%).
Figure 1: (A) Fluid Input, Output and Fluid Balance Changes at 12-Hour Intervals; and (B) Cumulative Fluid Input, Output and Fluid Balance.
Fluid Resuscitation
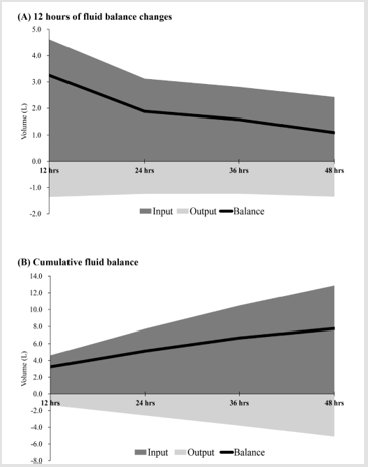
Table 2 depicts the results of logistic regression analysis of associations between fluid resuscitation and organ failure, inhospital mortality and 30-day mortality. Multivariate logistic regression analysis revealed that the odds of organ failure increased significantly in association with increased fluid input at 0-12 hours and 36-48 hours [adjusted odds ratio (aOR) = 1.125, 95% confidence interval (CI) = 1.019-1.243; aOR = 1.207, 95% CI = 1.0002-1.457], and with increased fluid balance at 0-12 hours and 36-48 hours (aOR = 1.103, 95% CI = 1.004-1.210; aOR = 1.180, 95% CI = 1.009-1.380, respectively). The odds of in-hospital mortality increased significantly associated with increased fluid input at 24- 36 hours (aOR = 1.205, 95% CI = 1.058-1.373) and with increased fluid balance at 24-36 hours (aOR = 1.196, 95% CI = 1.049-1.364). In addition, the odds of 30-day mortality increased significantly associated with fluid input increases at 24-36 hours (aOR = 1.175, 95% CI = 1.028-1.343) and with fluid balance increases at 24-36 hours (aOR = 1.188, 95% CI = 1.038-1.359) (Table 2).
Table 2: Logistic Regression Analysis of Fluid Resuscitation Associated with Organ Failure, In-Hospital Mortality and 30-Day Mortality.
aMultivariate logistic regression was simultaneously adjusted for age, gender, race, heart rate and white blood cells. (Temperature
and respiratory rate were not put into model adjustments because all organ failure patients have abnormal temperatures and almost
all patients have abnormal respiratory rates.)
bMultivariate logistic regression was simultaneously adjusted for age, gender, race, temperature and white blood cells. (Heart rate
and respiratory rate were not put into model adjustments because heart rate and respiratory rate are normal in survived patients.)
Cumulative Fluid Resuscitation
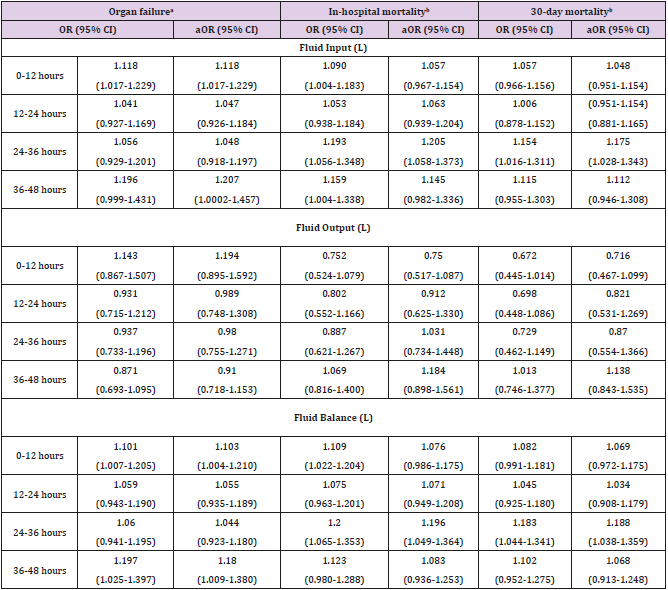
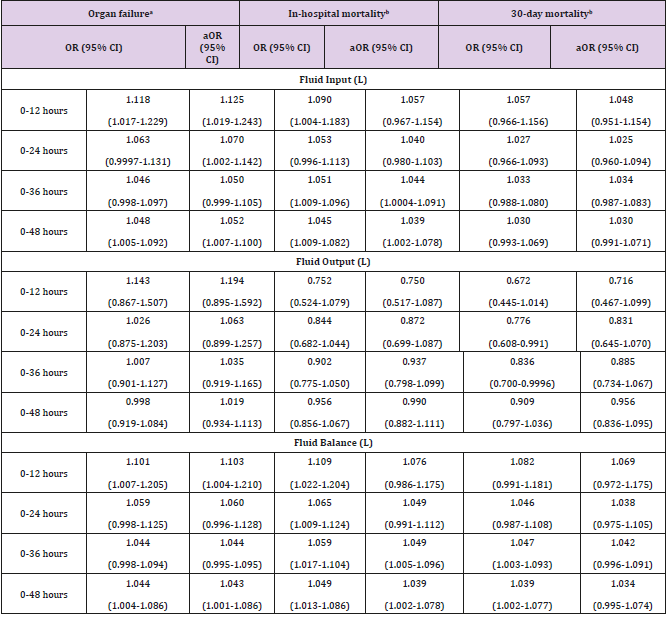
Table 3 depicts the results of multivariate logistic regression analysis of cumulative fluid resuscitation. The odds of organ failure increased significantly in association with increased fluid input at 0-12 hours, 0-24 hours and 0-48 hours (aOR = 1.125, 95% CI = 1.019-1.243; aOR = 1.070, 95% CI = 1.002-1.142; aOR = 1.052, 95% CI = 1.007-1.10, respectively). In addition, the odds of organ failure increased significantly associated with increased fluid balance at 0-12 hours and 0-48 hours (aOR = 1.103, 95% CI = 1.004-1.210; aOR = 1.043, 95% CI = 1.001-1.086, respectively). The odds of inhospital mortality increased significantly associated with increased fluid input at 0-36 hours and 0-48 hours (aOR = 1.044, 95% CI = 1.0004-1.091; aOR = 1.039, 95% CI = 1.002-1.078, respectively). Similarly, the odds of in-hospital mortality increased significantly in association with increased fluid balance at 0-36 hours and 0-48 hours (aOR = 1.049, 95% CI = 1.005-1.096; aOR = 1.039, 95% CI = 1.002-1.078, respectively). However, the odds of 30-day mortality decreased significantly in association with increased fluid output at 0-24 hours and 0-36 hours (OR = 0.776, 95% CI = 0.608-0.991; OR = 0.836, 95% CI = 0.70-0.9996, respectively), and the odds of 30-day mortality increased significantly associated with increased fluid balance at 0-36 hours and 0-48 hours (OR = 1.047, 95% CI = 1.003-1.093; OR = 1.039, 95% CI = 1.002-1.077, respectively). After adjusting for covariates, the results of multivariate logistic regression were no longer significant (Table 3).
Table 3: Logistic Regression Analysis of Cumulative Fluid Resuscitation Associated with Organ Failure, In-Hospital Mortality and 30-Day Mortality.
aMultivariate logistic regression was simultaneously adjusted for
age, gender, race, heart rate and white blood cells. (Temperature
and respiratory rate were not put into model adjustments
because all organ failure patients have abnormal temperatures
and almost all patients have abnormal respiratory rates.)
bMultivarate logistic regression was simultaneously adjusted
for age, gender, race, temperature, white blood cells and organ
failure. (Heart rate and respiratory rate were not put into model
adjustments because heart rate and respiratory rate are normal
in survived patients.
Discussion
The present study examined the influence of fluid resuscitation
on the occurrence of organ failure, in-hospital mortality and 30-
day mortality in 317 AP patients from the MIMIC III database
who had been in the ICU for at least 48 hours. We found mainly
that the amount of early fluid input (0-12 hours) or the cumulative
fluid resuscitation after 48 hours in ICU is associated with the
incidence of organ failure in these patients. The amount of fluid
input for 24 to 35 hours and the cumulative fluid resuscitation at
36 or 48 hours in ICU are associated with in-hospital mortality for
AP patients. Fluid balance showed similar effects as fluid input. In
the AP patient population studied, the amount of early fluid balance
(0-12 hr) or the cumulative fluid resuscitation at 48 hours in ICU
is also associated with the incidence of organ failure, and for 24 to
36 hours or the cumulative fluid balance at 36 or 48 hours in ICU
is associated with in-hospital mortality. However, the fluid output
appears to have no association with organ failure or mortality in
this patient population.
Severe AP involves inflammation of the pancreas combined
with SIRS causing extravasation of fluid into interstitial spaces,
which can lead to hypovolaemia, hypoperfusion and organ failure
[1,8]. To offset this projected course in the absence of curative
therapy, it becomes critical to provide supportive therapy with fluid
resuscitation and management of pain in the early acute phase [18].
However, even though early fluid resuscitation is reported to reduce
morbidity and mortality in AP patients, the agreement is lacking
on fluid type and amounts. Some authors advise first achieving
hemodynamic stability by bolusing fluids and then administering
250cc to 500cc of balanced crystalloid solutions in patients without
volume depletion and 500cc to 1000cc for those with severe
depletion [9]. The IAP/APA guideline based its recommendations
for fluid resuscitation on one randomized controlled trial that
showed improvements in the CRP levels and SIRS with the use
of Ringer’s lactate [8], and although other studies use balanced
fluids like Plasma-Lyte, the benefits of these fluids have not been
confirmed to date [1].
One study showed that patients receiving goal-directed fluid
therapy with invasive monitoring had shorter ICU stays, less
ventilator support, fewer incidents of compartment syndrome and
organ failure, and lower in-hospital mortality [18]. In contrast,
a randomized controlled trial with 115 patients found that
rapid, uncontrolled fluid resuscitation within 48 hours (or until
hematocrit was <35%) actually resulted in significant increases
in the rates of infection, abdominal compartment syndrome, need
for mechanical ventilation and mortality [15]. In the present study,
the amount of early fluid input (0-12 hours) was associated with
an increased incidence of organ failure, primarily in patients with
severe disease already accompanied by SIRS. Another investigation
of early fluid resuscitation of AP patients reported a reduced
incidence of SIRS and organ failure at 72 hours, but this was mainly
in patients with interstitial disease and not in severe cases [19].
Those authors concluded that early fluid resuscitation is most
beneficial in preventing severe disease in those admitted with less
severe AP.
Other authors found that effects of the initial intravenous fluid
resuscitation rate in the first 24 hours of admission increased the
risk of mortality in patients with severe AP unless the early fluid
resuscitation amount was at least one-third of the cumulative fluid
resuscitation amount at 72 hours [20]. In other words, aggressive
early treatment-more fluid at a faster rate- achieved more favorable
outcomes. In the present study, the amount of cumulative fluid
resuscitation after 48 hours in ICU was associated with an increased
incidence of organ failure, and the amount of fluid input for 24 to
35 hours and the cumulative fluid resuscitation at 36 or 48 hours
were associated with in-hospital mortality for AP patients. Among
our patient population, the odds of in-hospital mortality increased
significantly associated with increased fluid input at 24-36 hours
and with increased fluid balance in the same timeframe.
When equal numbers of AP patients in an “early” resuscitation
group and a “late” resuscitation group received the same total
volume of fluid over 72 hours but the early group received more
of that volume in the first 24 hours, the “late” group had a higher
rate of in-hospital mortality, with four patients dying of acute renal
failure, cardiopulmonary arrest and ventricular tachycardia [20].
However, rates of organ failure, SIRS development and hospital
length of stay were the same between the two groups. Results of
that study again support the idea of aggressive fluid resuscitation
in the first 24 hours after hospital admission, including volume and
rate. Accordingly, in the present study, since early fluid resuscitation
was associated with organ dysfunction but not with in-hospital or
30-day mortality, we suggest, along with other authors [19], that it
was the acute severe status of our AP patients that influenced organ
involvement and not the volume or rate of fluid resuscitation.
The present study found no association between fluid output
and organ failure or mortality.
Another study that used the large MIMIC III database found that,
in critically ill AP patients with negative fluid balance, increased
fluid intake and increased urine output were both associated with
lower risk of hospital mortality [21]. The authors of that study
believed that fluid intake was directly responsible for increased
urine output in their patient population, and that the association between urine output and mortality was secondary following an
association between fluid intake and mortality. They also suggested
that achieving a negative fluid balance in patients who are receiving
high fluid intake is a factor most likely related to satisfactory organ
function. The degree of AP severity, again, appears to greatly
influence outcomes of fluid resuscitation. In patients with severe
AP with SIRS and organ failure, the hour-to-hour administration of
fluids (fluid resuscitation profiles) are found to vary considerably
between those who survive and those who don’t [14], and standard
measures of judging adequate filling are needed. Although the
present study did not consider fluid monitoring parameters in this
investigation, it is clear that managing fluids in critically ill patients,
whether AP or other diagnoses, is a clinical challenge because of
the well-known associations between fluid overload and poor
outcomes [3].
As such, monitoring fluid resuscitation in AP is essential. The
IAP/APA guideline [8] recommends using specific target measures
to monitor fluid resuscitation since no single parameter indicates
hydration status; laboratory measures include hematocrit (ideally
between 35% and 44%), and blood urea nitrogen (BUN) and
creatinine levels (within the normal range); continuous monitoring
in the ICU includes heart rate (less than 120/min), mean arterial
pressure (between 64-85mmHg) and urinary output (0.5 ml/kg/
hour). Newer monitoring methods that focus on the intravascular
compartment include cardiac output monitoring and transesophageal
Doppler waveform analysis [14]. As monitoring
methods for fluid resuscitation in AP improve, it may shed light on
the most beneficial amounts and rates of fluid administration from
early fluid resuscitation to cumulative fluid resuscitation, helping to
refine fluid resuscitation profiles.
Strengths and Limitations
The present study used data retrieved from MIMIC III, a highquality database that provides demographic and clinical data from a large, diverse population of ICU patients. The quality of our data analysis and research results benefitted greatly from the high temporal resolution data of MIMIC III, including clinical laboratory results, electronic documentation, and bedside monitoring trends and waveforms. However, this study also has certain limitations, mainly that the data were derived from only one medical center, meaning that the study results cannot be generalized to other populations in other locations. Lifestyle and dietary information, environmental exposure and family medical history are not included in the MIMIC III database and therefore could not be analyzed; we must consider that our results may have been influenced by these factors if they had been included in the analysis. Fluid balance prior to ICU admission was also not included in the analysis since only limited information was included in the database, and this omission could lead to information bias. Also, only in-hospital data during patients’ ICU stays were included and no information was provided on medical utilization after discharge. Finally, due to the nature of retrospective research using a secondary database, only associations between fluid resuscitation and mortality could be inferred. Further prospective studies are needed to investigate the optimization of fluid resuscitation protocols associated with improved outcomes for severe acute pancreatitis.
Conclusion
In fluid resuscitation for ICU patients with AP and SIRS, amounts of early fluid input (first 12 hours) or cumulative fluid resuscitation within 48 hours are associated with organ failure. Amounts of fluid input from 24 to 36 hours or cumulative resuscitation after 36-48 hours are associated with in-hospital mortality, and fluid balance has similar effects. Fluid output has no association with organ failure or mortality in this patient population. Results of the present study suggest that early fluid resuscitation is essential in severe AP but that the entire hour-to-hour fluid resuscitation profile determines outcomes. Our results also emphasize the need for consensus on a classification system for AP severity, a monitoring protocol for use in nursing units to guide fluid resuscitation and standard recommendations for fluid administration, including fluid type, volume, and rate of administration associated with satisfactory outcomes.
Declarations
Ethical Considerations and Consent to Participate
Use of the MIMIC-III database is approved by the Institutional Review Boards of Beth Israel Deaconess Medical Center (BIDMC), Boston, MA, USA, and Massachusetts Institute of Technology and use of the database is granted to investigators for research purposes. Since all patient data in MIMIC III database is de-identified, signed informed consent by patients is not required.
Data Availability
The MIMIC-III database is freely accessible. Use of the database by researchers is granted by the Institutional Review Board of Beth Israel Deaconess Medical Center (BIDMC) for research purposes only.
Consent for Publication:
Not applicable.Competing Interests
The authors have no conflicts of interest to declare associated with the present study.
Funding
None.
Author Contributions
[please make a list using authors’ initials (e.g., LLC) with project description such as Subject selection; Data analysis; Writing manuscript; Manuscript evaluation; etc.]
Acknowledgment
The authors wish to the Philips Healthcare and staff at the Beth Israel Deaconess Medical Center, Boston, for supporting database development.
References
- van Dijk SM, Hallensleben NDL, van Santvoort HC, Fockens P, van Goor H, et al. (2017) Acute pancreatitis: recent advances through randomised trials. Gut 66(11): 2024-2032.
- Dellinger EP, Forsmark CE, Layer P, Lévy P, Maraví-Poma E, et al. (2012) Determinant-based classification of acute pancreatitis severity: an international multidisciplinary consultation. Ann Surg 256(6): 875-880.
- Johnson CD, Besselink MG, Carter R (2014) Acute pancreatitis. BMJ 349: g4859.
- Lowenfels A, Maisonneuve P, Sullivan T (2009) The changing character of acute pancreatitis: epidemiology, etiology and prognosis. Curr Gastroenterol Rep 11(2): 97-103.
- Janisch NH, Gardner TB (2016) Advances in Management of Acute Pancreatitis. Gastroenterol Clin North Am 45(1): 1-8.
- Roberts SE, Morrison-Rees S, John A, Williams JG, Brown TH, et al. (2017) The incidence and aetiology of acute pancreatitis across Europe. Pancreatology 17(2): 155:165.
- Janisch NH, Gardner TB (2016) Advances in management of acute pancreatitis. Gastroenterol Clin North Am. 45(1):1-8.
- Working Group IAP/APA Acute Pancreatitis Guidelines (2013) IAP/APA evidence-based guidelines for the management of acute pancreatitis. Pancreatology. 13(Suppl 2): e1-15.
- Tenner S, Baillie J, DeWitt J, Vege SS (2013) American College of Gastroenterology. American College of Gastroenterology guideline: management of acute pancreatitis. Am J Gastroenterol 108(9): 1400-1415.
- Wu BU, Hwang JQ, Gardner TH, Repas K, Delee R, et al. (2011) Lactated Ringer’s solution reduces systemic inflammation compared with saline in patients with acute pancreatitis. Clin Gasteroenterol Hepatol. 9(8): 710-717e-1.
- Du XJ, Hu WM, Xia Q, Huang ZW, Chen GY, et al. (2011) Hydroxyethyl starch resuscitation reduces the risk of intra-abdominal hypertension in severe acute pancreatitis. Pancreas 40(8): 1220-1225.
- Kobayashi H, Takahashi O, Fujita Y (2017) Fluid balance and organ failure in acute pancreatitis: retrospective cohort study. Pancreas 46(5): e47-e49.
- Aakash Aggarwal, Manish Manrai, Rakesh Kochhar (2008) Fluid resuscitation in acute pancreatitis. Clin World J Gastroenterol 20(48): 18092-18103.
- Mole DJ, Hall A, McKeown D, Garden OJ, Parks RW (2011) Detailed fluid resuscitation profiles in patients with severe acute pancreatitis. HPB (Oxford) 13(1): 51-58.
- Mao EQ, Tang YQ, Fei J, Qin S, Wu J, et al. (2009) Fluid therapy for severe acute pancreatitis in acute response stage. Chin Med J (Engl) 122(2): 169-173.
- Aggarwal A, Manrai M, Kochhar R (2014) Fluid resuscitation in acute pancreatitis. World J Gastroenterol 20: 18092-18103.
- Johnson AE, Pollard TJ, Shen L, Lehman LH, Feng M, et al. (2016) MIMIC-III, a freely accessible critical care database. Sci Data 3: 160035.
- Wang MD, Ji Y, Zu J, Jiang OH, Luo L, et al. (2013) Early goal-directed fluid therapy with fresh frozen plasma reduces severe acute pancreatitis mortality in the intensive care unit. Chin Med J (Engl) 126(10): 1987-1988.
- Warndorf MG, Kurtzman JT, Bartel MJ, Cox M, Mackenzie T, et al. (2011) Early fluid resuscitation reduces morbidity among patients with acute pancreatitis. Clin Gastroenterol Hepatol 9(8): 705-709.
- Gardner TB, Vege SS, Chari ST, Petersen BT, Topazian MD, et al. (2009) Faster rate of initial fluid resuscitation in severe acute pancreatitis diminishes in-hospital mortality. Pancreatology 9(6): 770-776.
- Shen Y, Huang X, Zhang W (2017) Association between fluid intake and mortality in critically ill patients with negative fluid balance: a retrospective cohort study. Crit Care 21(1): 104.

 Research Article
Research Article