Background
In 2011 Dr. Bril and her associates from three major specialties published an exhaustive study of all the published treatments for painful peripheral neuropathy (PPN) from 1960 until July of 2008 and concluded that certain pharmaceuticals met Class I evidence-based standards for treating PPN [1]. One year later Dr. Bril noted that pharmaceuticals did not help the majority of PPN patients who received them, had significant adverse side effects and that “interventions aimed at nerve regeneration may need to be employed” [2].
In 2015, Finnerup [3] and colleagues performed a systematic review and meta-analysis of the data describing pharmacotherapy for neuropathic pain for the IASP; they concluded that the “inadequate response to drug treatments constitutes a substantial unmet need in patients with neuropathic pain.” In 2016, Richard Rosenquist, MD of the Cleveland Clinic presented to the ASRA meeting his systematic review and meta-analysis of the available data concerning the pharmacologic treatment for peripheral neuropathy and concluded that it was “miserable, … frustrating, … and maybe even appalling” [4].
In 2017, the Cochrane [5] group released a meta-analysis of 37 Class I studies that covered 5914 participants who received high dose gabapentin to treat their PPN. They documented that less than 60% of the patients reduced their pain by 50% or more while over 60% had adverse side effects. Experts in the field of evidence based medicine state that “Real evidence-based medicine makes the ethical care of the patient its top priority” [6]. Hippocrates teaches the basic ethical principle of the Art of Medicine when he states “As to diseases, make a habit of two things -- to help, or at least to do no harm”. A large number of Class I studies show that pharmaceuticals treat PPN better than placebo; given the dismal results, how can we then claim to practice medicine ethically when our treatments help less than 60% of patients while harming more than 60% of patients who receive them? The World Medical Association Declaration of Helsinki states: “in the treatment of an individual patient, where proven interventions do not exist or known interventions have been ineffective, the physician after seeking expert advice, with the informed consent from the patient or legally authorized representative, may use an unproven intervention if in the physician’s judgement it offers hope of saving life, reestablishing health, or alleviating suffering. This intervention should subsequently be made the object of research, designed to evaluate its safety and efficacy. In all cases, new information must be recorded and, where appropriate, made publicly available [7].” My goal in this short article is to make this information publicly available. This small case review/editorial review data from two private practices which document how an “unproven intervention” offers a safe and effective way to treat PPN. As such it meets the WMA Declaration of Helsinki request to record and evaluate its safety and efficacy.
Introduction
Drugs do not adequately treat the symptoms of painful neuropathy or modify the underlying nerve damage seen with neuropathy. Because the pharmaceutical treatments available for painful peripheral neuropathy (PPN) “do not relieve pain completely in the majority of patients and most have significant adverse effects” experts have suggested that “interventions aimed specifically at nerve regeneration may need to be employed [1].” RA Malik, co-author of the ADA Standards of Medical Care in Diabetics 2017 has recently noted that “current drugs have no benefit for the underlying nerve damage. We have witnessed failure after failure of clinical trials of disease-modifying drugs [8]”.
Accordingly, since drugs do not adequately treat the symptoms of neuropathy or repair the nerve damage caused by neuropathy, a different approach must be considered. As ascribed to Einstein: “We can’t solve problems by using the same kind of thinking we used when we created them.” Fortunately, his colleague, Erwin Schrodinger gave us a clue for a new approach when he taught: “Living matter at the cellular level can be thought of in terms … of pure physics [9]”. Odell and Sorgnard found that electronic signal therapy (EST) has profound anti-inflammatory effects [10]. This discovery has led these researchers to show that Combined Electrochemical Therapy (CET), which utilizes EST, can successfully treat diabetic (and all other forms of) neuropathy [11,12]. Carney presented an award winning poster (with a subsequent publication) at the American Academy of Pain Management which showed that “Quantum Theory Treats Neuropathy Better than Pharmacology [13]”. These promising results suggest that the electromagnetic fields used in EST and CET play a role in regenerating nerves. If, indeed, the nerves of patients suffering from neuropathy could be regenerated, then perhaps their lives could be improved.
Study Results
A poster presentation at the 2017 AAPM Meeting [14] described how 14 patients with five different types of PPN responded to CET in two private practice clinics [15]. Epidermal Nerve Fiber Density (ENFD) biopsies have been shown to be a new gold standard in evaluating neuropathy [16]. All patients had ENFD done before starting treatment and on average 4.5 months after ending treatment (0-9 months).
Eleven of the 14 patients (79%) had evidence of nerve regeneration at one or more sites. Eighteen of the 34 sites (53%) showed some growth, 7 (21%) showed no growth and 9 (26%) showed a decrease in the number of nerves. On average, each of the 18 positive sites went from initially having 2.7 fibers/mm to having 4.7 fibers/mm after treatment, a 74% increase in nerve fibers after receiving CET treatment. One patient with DPN went from having no fibers in her foot at the beginning to having a normal number (3.4 fibers/mm) after treatment. Her NRS went from 9/10 at the beginning to 2/10 at the end of treatment and was 3/10 at 38 months after treatment. She improved her NFI by 50% at the end of treatment and by 79% when last seen 38 months after treatment.
The average high VAS score during treatment was 7.7/10 and at the end of treatment 2.1/10 (average VAS decreases of 73% per patient). Eleven of 14 (79%) reduced their pain by at least 50% and all 14 (100%) reduced their pain by at least 40% at the end of treatment. The average highest NFI for all 14 patients receiving treatment was 51% compared to 24% at the end of treatment for an average improvement in function of 53%. Nine patients (64%) improved their function by 50% or more and 13 (93%) improved their function by at least 30%.
Data was available on the use of medication for 9 patients (64%). Of these nine patients, eight stopped one or more drugs that they were taking before treatment. Regarding opioid usage, four patients took opioids before treatment and three who were followed for an average of 35 months after finishing their treatment had an average reduction in their opioid use of 70% (50-100%). Three of four patients on pregabalin stopped their pregabalin altogether and two of three patients of gabapentin stopped it altogether and one had no change in its use. One patient stopped her 60mg/day of duloxetine.
None of these 14 patients had any adverse side effects.
Illustrative Case Report
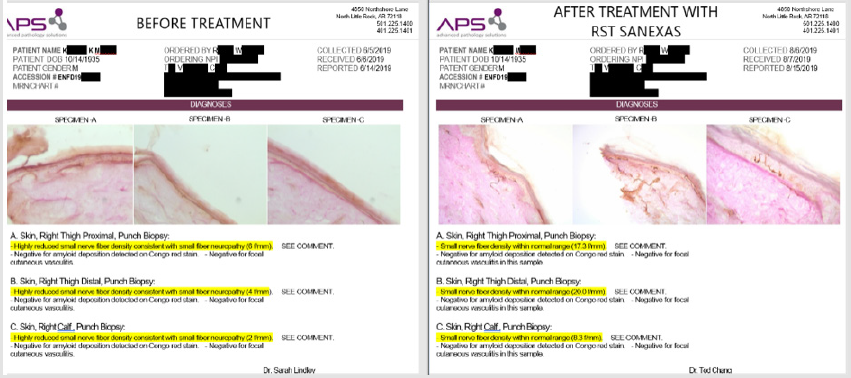
More and more clinics nationwide are utilizing ENFD biopsies to track patients treated with EST and CET. Figure 1 shows a recent example of nerve regrowth in a patient treated with the CET protocol at a third clinic. A comparison of the pre- and post-ENFD biopsies shows nerve regrowth in the proximal and distal thigh to normal values, while there is evidence of nerve regrowth increase in the left calf from no fibers to 2.7 f/mm, although this value is still below normal.
Discussion
For the last six years systematic reviews and meta-analysis of the use of drugs to treat painful peripheral neuropathy (PPN) have documented that drugs help less than half the patients who use them and have significant side effects [2,14,17]. Expert cited above have described drug therapy as “inadequate…frustrating… maybe even appalling [3].” and the Cochrane Study [4] mentioned above also validated this insight. Some authors have suggested that regenerating nerves may be needed to find an adequate, effective and safe treatment for PPN; however, clinical trials have produced failure after failure with no current benefit [7]. This small study and case report conclusively document that using electromagnetic energy fields via a technique called CET (Combined Electrochemical Therapy) have allowed a large majority of patients (79%) to regenerate one or more of their epidermal nerves. All patients had a decrease in their pain scores. A large majority of improved their function by the end of treatment. Eight of nine patients stopped or reduced their medication use. No patients had any adverse side effects.
Conclusion
While small in numbers, these outcomes show that using the principles of physics rather than pharmacology provides safer and more effective outcomes. The use of electromagnetic energy fields in combination with local anesthetics has allowed 79% of patients to regenerate nerves. Regenerating nerves was associated with decreased pain, improved function, reduced medication use and had no adverse side effects. As such this technique offers a safe and effective new way to treat painful peripheral neuropathy.
Acknowledgement
The author offers his deepest thanks to Peter Carney, MD, whose vision has long guided our efforts, and Richard Sorgnard, PhD, who developed the advanced electromagnetic device used in this study.
Conflict of Interest
Robert Odell, MD, PhD: Owns stock in the company, RSTMed, which distributes the device used in this study.
References
- Bril v, England J, Franklin GM, Backonja M, Cohen J, et al. (2011) Evidence-based guideline: Treatment of painful diabetic neuropathy: report of the American Academy of Neurology, The American Association of Neuromuscular and Electrodiagnostic Medicine, and the American Academy of Physical Medicine and Rehabilitation. Neurology 76(20): 1758-1765.
- Vera Bril (2012) Treatment for Diabetic Neuropathy. Journal of the Peripheral Nervous System 17(s2): 22-27.
- Finnerup NB, Atta N, Haroutian S, McNicol E, Baron R, et al. (2015) Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol 14(2): 162-173.
- Rosenquist, Richard (2017) Meeting of the American Society of Regional Anesthesia and Pain Medicine: 2016. As quoted in PAINMEDICINENEWS
- Wiffen PJ, Derry S, Bell RF, Rice ASC, Tolle T, et al. (2017) Gabapentin for chronic neuropathic pain in adults. Cochrane Database of Systematic Reviews 9(6).
- PLACEHOLDER – NEED TO FIND
- (2018) WMA Declaration of Helsinki-Ethical Principles for Medical Research Involving Human Subjects World Medical Association.
- Malik, RA. (2017) quoted in Medscape Medical News Jan. 09, 2017
- Schrodinger E (1943) What is Life? The Physical Aspect of the Living Cell. Lectures given Trinity College, Dublin, Ireland Feb 1943. Printed Cambridge University Press. 1944.
- Odell RH Jr, Sorgnard RE (2008). Anti-Inflammatory Effects of Electronic Signal Treatment. Pain Physician 11(6): 891-907.
- Cynthia Cernak, Elizabeth Marriott, John Martini, Jeremy Fleischmann, Briana Silvani, et al. (2012) Electrical Current and Local Anesthetic Combination Successfully Treats Pain Associated with Diabetic Neuropathy. Practical Pain Management 12(3).
- Cynthia Cernak, Robert H Odell, Peter Carney (2014) Can Combined Electrochemical Treatment Have an Impact for Diabetic Peripheral Neuropathy? Podiatry Today 27(7): 20-24.
- Carney P (2014) Quantum Theory Treats Neuropathy Better Than Pharmacology. The Pain Practitioner 24(4): 28-31.
- (2017) AAPM 2017 Annual Meeting Abstracts. Pain Medicine 18(3): 569-621.
- Because neither of these practices were associated with medical institutions, Institutional Review Board (IRB) certification was not possible. However, this study, presented at the March, 2017 AAPM meeting conforms to the AAPM standards for poster presentations by obtaining informed consent from each patient in accordance with the “WMA Declaration of Helsinki – Ethical Principles for Medical Research Involving Human Subjects.” Adopted by the 18th WMA General Assembly, Helsinki, Finland, June 1964, and amended by the 64th WMA General Assembly, Fortaleza, Brazil, October 2013: Section 37.
- David S Saperstein, Todd D Levine (2009) Diagnosing Small Fiber Neuropathy Through the Use of Skin Biopsy. Practical Neurology 37-40.
- Moulin D, Boulanger A, Clark AJ, Clarke H, Dao T, et al. (2014) Pharmacologic management of chronic neuropathic pain: Revised consensus statement from the Canadian Pain Society. Pain Res manag 19(6) 328-335.

 Case Report
Case Report