Abstract
Many acquired (cerebrovascular stroke, traumatic brain injury) and progressive neurological conditions (Parkinson’s disease, ALS) manifest observable impairments in movement, while changes in somatosensory function may be inconspicuous and more difficult to measure. Research trials and clinical study of movement disorders would benefit from a device and control software that can be used to assess an individual’s cutaneous sensitivity to highly controlled mechanical stimulation over a range of test frequencies, similar in concept to pure tone behavioral audiometry. In the present report, we describe the implementation of an automatic single-interval up/down (SIUD) adaptive procedure to estimate vibrotactile detection thresholds (VDT) of the dominant glabrous hand and homolateral perioral skin in response to sinusoidal mechanical stimuli presented at 5, 10, 50, 150, 250 and 300 Hz. The resulting VDT function, herein known as a vibrogram, can be acquired in approximately 3 minutes 45 seconds for any given skin site.
An embedded Field Programmable Gate Array (FPGA) microcontroller (cRIO National Instruments) operating under MS Windows 8.1 is used for real-time stimulus generation and randomization of test frequencies, adaptive stimulus amplitude control, threshold response data visualization, and automated logging of the subject’s threshold results to an Excel file. This new system was tested on the lower face and glabrous hand in a cohort of 89 neurotypical adults which revealed that VDT’s were significantly dependent on stimulus site (F=248.64, p<.0001), and frequency of vibrotactile stimulation (F=65.19, p<.0001). Sex of the subject was not a significant factor (F=0.26, p=.612). The differences in the VDT’s between the hand and face are presumed to reflect the unique typing and distribution of mechanoreceptors in the face and hand, and differences in the integument at these two sites. The increased sensitivity (classic U-function) at 250Hz, attributable to the presence of the rapidly adapting Pacinian corpuscles in the glabrous hand, was not apparent in the perioral vibrogram. The automation of our VDT tracking algorithm modeled after Lecluyse and Meddis, provides clinical investigators with a reliable tool for rapid assessment of the cutaneous somatosensory system on both glabrous and hairy skin in humans across the lifespan.
Keywords: Adult; Somatosensory; Automatic Adaptive Cutaneous Threshold Tracking; Glabrous; Nonglabrous
Introduction
The face and hand convey our identity, manipulate and interact with the environment, and express emotion and communicative intent. The skin covering these structures with its intricate net of neurites and mechanosensory endings also serves as a receiver of tactual (haptic) information for detection and recognition of touch, shape, texture, pleasure, consequences of movement, and to alert us of potential environmental hazards. An intact integumentary system also provides a safe environment for our bodies through a bidirectional barrier accomplished by the epidermis to protect and reproduce our DNA [1]. In many acquired and progressive diseases however, somatosensory impairment often leads to higher mortalities due to limited activity and increased risk of injury. Not only is there a disruption of tactile reception and interpretation, there is often a deficit in coordinated motor performance due to a loss of feedback mechanisms necessary for stereognosis, kinesthesia, and movement related proprioception. An estimated one in two stroke survivors have enough loss of the sense of touch that they have difficulty with everyday activities [2]. Ischemic damage to cortical and subcortical regions in stroke results in degraded motor execution and management of somatosensory signals from peripheral receptors [3,4].
Additionally, there is widespread disruption of excitatory and inhibitory networks responsible for somatosensory, executive and visuospatial processing [5-6]. The resulting impairment can lead to life-long sensory sequelae and contributes to functional deficit and hemispatial neglect [7-9]. In another acquired disorder, traumatic brain injury, elevated intracranial pressure [10,11], and axonal torque injury [12] can lead to long-term somatosensory impairment [13-15]. As with cerebrovascular stroke, the extent of somatosensory deficit can be difficult to identify in many of these patients.
Some neurodegenerative diseases have a negative impact on somatosensation, including Parkinson’s disease (PD), in which primary dopaminergic denervation of the basal ganglia is associated with abnormalities in vibrotactile thresholds, object discrimination, and pain sensitivity [16-19]. Parkinson’s-related movement ‘freezing’ may be due to reduced connectivity to Supplementary Motor Area (SMA) and pre-SMA, altering Somatosensory Temporal Discrimination Thresholds (STDT) that affect motor production and timing [20,21]. Moreover, continuous adjustments to dopaminergic treatments and evolving degeneration results in a constant fluctuation of somatosensory symptoms that may require longterm monitoring [22,23].
Like PD, the progression of amyotrophic lateral sclerosis (ALS) may degrade somatosensory integrity. Even though ALS is predominately characterized by motor neuron degeneration, peripheral and central degeneration of sensory pathways can occur, particularly in early stages of the disease [24-27]. Non-motor progression as evidenced by spinal imaging of the dorsal column [28] and sensory nerve biopsy [29], might be more readily assessed in these patients using non-invasive somatosensory threshold testing in the clinical environment. Arguably even more common in human health issues, changes in peripheral and central vascularization such as those seen in diabetes and heart disease, greatly affect somatosensation and the integrity of the integumentary system [30-33]. As in neurological disease, poor tactile acuity due to problems with peripheral circulation should be evaluated over the course of therapeutic intervention and disease progression. Despite the importance of measuring the integrity of somatosensory pathways in damaged systems, the availability of non-invasive, time-efficient assessment methods are limited. In clinical settings it can be difficult to evaluate specific somatosensory impairment or ascertain how much somatosensory damage is contributing to problems with motor performance [34-36].
From a long-term care perspective, rehabilitation research has shown that compromised somatosensory function is related to longer length of stays for institutionalized patients [37,38] and lower quality of life ratings from patients who are living at home [39,40]. Thus, the goal of the present study was to describe our design and implementation of a an automatic single-interval up/down (SIUD) adaptive procedure and microprocessor-based hardware control system to estimate vibrotactile detection thresholds (VDT) of the dominant glabrous hand and homolateral perioral skin in response to sinusoidal mechanical stimuli presented at frequencies ranging from 5 to 300 Hz in a cohort of young adults. We hypothesized this automated (3min-45sec) SIUD VDT procedure would reveal significant differences in vibrotactile threshold as a function of site (hand versus face) and stimulus frequency.
Materials and Methods
Participants
Eighty-nine (89) neurotypical adults (59F/30M [24.33 (SD=5.68) years] were recruited regardless of race or ethnicity. Written informed consent, approved by the university Institutional Review Board, was obtained for each participant. Participants were compensated for their participation in this study. Eightysix adults reported right-hand dominance, and 3 reported lefthand dominance. Inclusion criteria: no report of neurological or psychiatric illness, and not taking regular medication. Exclusion criteria: Neurological, sensory and/or muscular deficits, psychiatric abnormalities, trauma to face and/or hand, or with abnormal skin sensitivity on face or hand.
Vibrotactile Detection Threshold (VDT) Assessment for Hand and Face Stimulus Control
A linear electrodynamic exciter motor (Brüel & Kjaer model 4810 Minishaker, +/- 3mm displacement range) controlled by our software (VIBROS) was used to assess cutaneous vibrotactile sensitivity in the lower face and hand. Adaptive stimulus control was achieved using a National Instruments cRIO real-time FPGA embedded controller programmed in LabVIEW to synthesize (NI 9263, 16-bit, 100KS/s) 1-second sinusoidal bursts followed by an off-state for 1-second. A linear rise-fall decay function of 100 ms during burst generation circumvented mechanical transients associated with the on/off waveform transitions. This voltage signal was conditioned by a Brüel & Kjaer model 2706 power amplifier and input to the motor. The Minishaker includes custom fixtures and an integral Schaevitz subminiature Differential Variable Reluctance Transformer (DVRT) sensor to transduce displacement for precision vibrotactile stimulation and measurement, a stainless- steel shaft and nylon contactor probe (Area = 0.5 cm2) and a stainless-steel rigid surround (annular gap = 1 mm).
The probe-surround was coupled to a linear micrometer translation stage that was used for actuator displacement calibration and skin contactor preload. This fixture configuration allows the surface of the rigid surround to be adjusted relative to the contactor probe to produce a 500μm tissue preload against the moving stimulator probe. The DVRT displacement sensor provided an output signal linearly related to contactor probe displacement from DC to 800 Hz (resolution 0.01 μm). Participants were seated in a medical-grade hydraulic examination chair with an articulating headrest and a height-adjustable worktable and asked to press a response button as soon as they detected the vibratory stimulus. The Minishaker was coupled to a wall-mounted Zeiss operating microscope arm which provided for stable positioning near the target skin surface (hand or face). The orientation of the motor’s probe-surround to the glabrous finger and oral angle is shown in Figures 1 & 2. A double adhesive collar (7/16” ID) was placed on the stainless-steel surround fixture of the Minishaker to secure placement of the probe on the skin.
Adaptive Vibrotactile Threshold Tracking Algorithm: A single-interval up/down (SIUD) adaptive procedure originally described by Lecluyse and Meddis [41] for assessing auditory function, was incorporated into our somatosensory research to estimate vibrotactile thresholds at 5, 10, 50, 150, 250 and 300 Hz on the glabrous surface of the distal phalanx of the dominant index finger, and at the homolateral nonglabrous surface of the oral angle. These sinusoidal vibrotactile inputs correspond to the frequency sensitivity of cutaneous mechanoreceptors innervating the face and hand. Test order for site and stimulus frequency was randomized among participants. Participants wore circumaural headphones with narrow-band noise plus a continuous pure tone (70 dB SPL) centered at the active test frequency to mask the potential acoustic emittance associated with the Minishaker.
Participants were instructed to press a response button when they perceived ‘felt’ the vibratory stimulus. We briefly describe our adaptation of the Lecluyse and Meddis algorithm below. The initial stimulus amplitude for any given stimulus frequency was set at a supra-threshold level in order to ensure a detection response. The initial step size was set at 10 dB, and then randomly varied in a ± 5 dB range relative to the initial amplitude. After the first negative response, the stimulus level was set at the mid-point between the previous 2 levels, and a 2dB step was subsequently utilized. The VDT test procedure continued for 8 trials starting from the trial prior to the first negative response. The algorithm implemented in this study also used false positive detection tests (foils) in which no vibrotactile stimulus was presented to ensure participant vigilance. These false positive trials were implemented in 20% of the successive trials, and on software detection of a false positive trial, it was discarded, and a new trial was initiated. The number of trials (n = 8) included in threshold estimation was chosen in order to attain an accuracy of ± 2 dB and this number excludes the false positive trials which typically extend any given threshold run by 1 or 2 additional trials [42].
Statistical Analysis
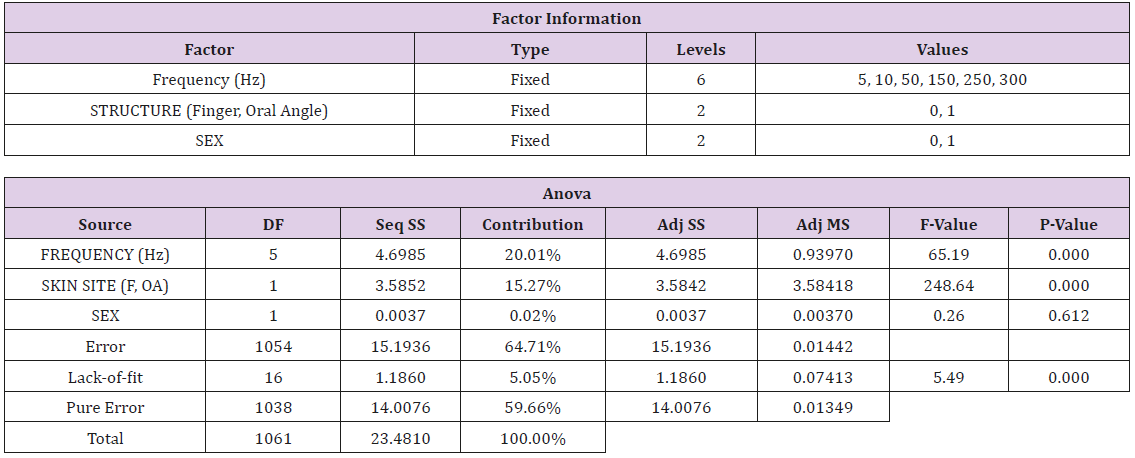
A repeated measures general linear model (GLM) using the factors Stimulus Frequency [6 levels: 5, 10, 50, 150, 250, 300 Hz], Skin Site [2 levels: glabrous index finger distal phalanx, oral angle hairy skin], and Sex [2 levels: Female, Male] was performed using Minitab ® v17.
Results
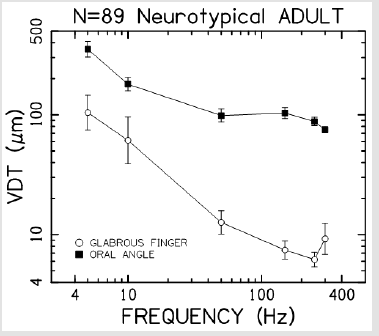
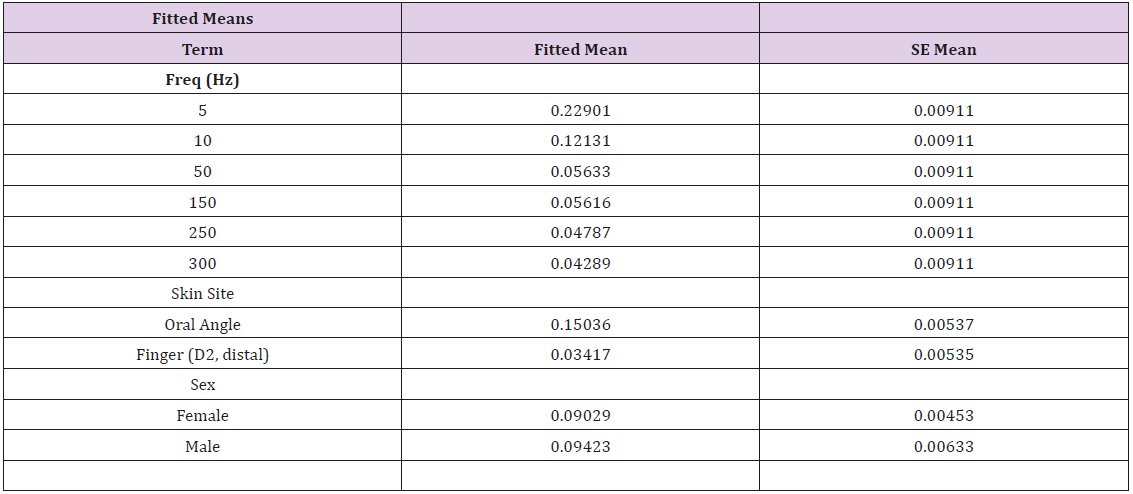
Vibrotactile thresholds were obtained for the glabrous skin of the index finger and hairy face in all 89 participants in less than 4 minutes per structure using the SIUD adaptive threshold procedure. The GLM statistical analysis revealed VDT’s were significantly dependent on stimulus site (F=248.64, p<.0001) and frequency of mechanical stimulation (F=65.19, p<.0001). Sex of the subject was not significant (F=0.26, p=.612). As shown in Figure 3, VDTs pooled among all subjects revealed that the glabrous finger is most sensitive and shows the classic U-function attributed to the PC-response at 250 Hz. The hairy facial skin site at the oral angle lacked the PC-response at 250 Hz. A summary of the GLM ANOVA and the estimated fitted means are given in Tables 1 and 2, respectively
Figure 3: Vibrotactile threshold functions for the glabrous index finger tip, and lateral face at the oral angle (hairy skin) based on 89 neurotypical adults.
Discussion
We developed and tested a new application known as VIBROS, using the SIUD adaptive procedure originally described by Lecluyse and Meddis [41] for auditory psychophysics, to estimate vibrotactile detection thresholds of the hairy skin at the oral angle and the glabrous surface of the distal (P4) phalanx of the glabrous index finger. This methodology represents a significant advance to our original report of mechanical frequency detection thresholds in the human hand and face that employed a time-consuming, hardware- intensive, manually executed method-of-limits [43] and extends preliminary work in our laboratory using this SIUD method in neurotypical elder cohorts [42]. The 3-minute 45 second SIUD procedure for the 6 test frequencies selected is efficient as it requires a significantly lower number of trials [41] in order to yield thresholds similar to those attained by traditional methods like the two-interval forced-choice, two-down/one-up and maximum-likelihood procedures.
The number of trials used for VDT estimation in this study (n = 8) did not include the false positive or ‘catch’ trials, and based on the mathematical model published earlier [41] this VDT estimation yields ± 2 dB accuracy. The significant differences in VDT’s between the 2 stimulus sites (finger, oral angle) among the neurotypical adults in the present study are consistent with known differences in the representation of mechanoreceptor typing and integument, and result in distinctly unique threshold detection profiles across the vibration test frequencies from 5 to 300 Hz. The present results also are in agreement with microneurographic recordings [44,45] and psychophysical studies in humans [42,43,46], which showed that mechanoreceptive afferents innervating the human perioral facial skin lacked Pacinian-type sensitivity at 250 and 300 Hz [47]. Dependence of VDTs on stimulus frequency and skin site is consistent with the response dynamics and threshold sensitivity of Aβ mechanoreceptors and unique relations of hairy versus glabrous skin with the surrounding integument. There were no significant differences for hand or face VDTs as a function of sex which is consistent with previously published studies [42,48,49].
A summary of these distinguishing anatomic and physiological properties which influence the contour and absolute VDT sensitivity are detailed in the following sections along with several new applications in haptics which benefit from VDT measurement tools. Skin receives a wealth of stimuli from the environment (touch, stretch, vibration, texture, pressure, itch, heat, cold, and pain) which are encoded by Aβ mechanoreceptors located in its layers [1]. Conformational changes to skin during imposed or voluntary movements also results in mechanoreceptor activity and a stream of somatosensory flow along peripheral nerves to the central nervous system [50]. Cutaneous receptors are classified into three groups according to response modality, including (1) mechanoreceptors for stretch, vibration, pressure and touch, (2) nociceptors for pain, and (3) thermoreceptors for heat (unmyelinated C-fibers) and cold (C-fibers and thinly myelinated Aδ fibers). Receptors are either unencapsulated or encapsulated and are in various levels of the skin. Morphology varies from simple naked nerve endings to complex structures [1].
Unencapsulated receptors include free nerve endings, Merkel disc (tactile discs) [51,56] and peritrichial nerve endings at the base of hair follicles. Encapsulated mechanoreceptors include Meissner corpuscles [57-59], Pacinian corpuscles, Krause end bulb corpuscles (temp, pressure especially in lips, tongue), and modified- Ruffini corpuscles [58-61]. Orofacial. The skin surrounding the mouth, cheeks and forehead is derived from the neural crest, and is soft and thin (4-layers), with fine hair and many sebaceous and eccrine sweat glands providing a somewhat oily texture, especially in women [1].
The epidermis ranges in thickness from 0.07 to 0.12 mm and is dominated by keratinocytes interspersed with Merkel cell mechanoreceptors, melanocytes, and other nonepithelial cells. In men, this skin lacks oil but forms many coarse hairs in the chin, cheek, and anterior parts of the lips. The glabrous lip vermillion is devoid of eccrine sweat glands and also thin (~1 mm) [1]. Unlike limb muscles, the muscles of facial expression, including the perioral and buccal anatomy, terminate in the dermis. The glabrous lips, perioral hairy skin, oral mucosa, and anterior and dorsum of tongue contain a high density of mechanoreceptors associated with rapidly conducting A β myelinated axons which are responsive to subtle mechanical deformation applied to their receptive fields [62]. The high innervation density of these Aβ mechanoreceptors is associated with high cortical magnification, defined as the ratio between the areas of representation in the primary somatosensory cortex (S1) to the area of the skin [63]. Perioral skin is predominantly populated with slow-adapting (SA) mechanoreceptors with small receptive fields (2-3 mm) which are well suited to encode facial movements, whereas the tongue tip is dominated by fast-adapting (FA) mechanoreceptors with even smaller receptive fields (~1 mm) [45]. Nordin and Hagbarth [60] described the response characteristics of 84 low-threshold mechanoreceptive afferents innervating facial hairy skin or glabrous lip sampled with microelectrodes from the human infraorbital nerve and found innervation density was highest near the corner of the mouth and on the upper lip.
The activity patterns of single primary SA afferents with large receptive fields were influenced by tangential skin stretch associated with voluntary contraction of facial muscles [60]. These findings suggest that trigeminal mechanoreceptors in hairy and glabrous perioral skin can contribute to facial kinesthesia and proprioception for motor control by signaling small variations in stretching and contraction [50,64]. Hand. In contrast to the face, the skin of the palms and soles, derived from the lateral plate mesoderm, is relatively thick (5-layers, 5+ mm) and glabrous with many eccrine sweat glands, and has individualized sole- and fingerprints that remain unchanged over a lifetime [1]. These skin areas also have a thicker epidermis (0.8 mm on palmar, 1.4 mm on soles) and are designed to tolerate friction and pressure and modulate stiction on smooth surfaces using the sweat glands.
The dermis is approximately 3 mm thick in the palm and tends to be thicker in men than in women. The papillary layer is a thin layer of dermis located immediately beneath the epidermis, and it covers the dermal papillae. The mechanoreceptors of encapsulated Meissner corpuscles reside in some of these papillae and are proximal to the basal lamina. They are sensitive to tactile stimuli of slight deformations in the epidermis and are particularly numerous in the lips, external genitalia, and nipples [1]. Merkel cells are intraepidermal mechanoreceptors that are scarcely distributed in adult skin but are numerous in the glabrous fingertips. Individual Merkel cells are found in close contact with an unmyelinated afferent nerve terminal (nerve plate) and manifest acute sensitivity to light touch [52,65]. They are derived from neural crest cells and another source of epidermal origin [1].
The encapsulated Pacinian corpuscle (PC) is the largest mechanoreceptor found in the dermis close to the hypodermis. Its end organ consists of fluid-filled concentric lamellae which form a capsule over the axon terminal [66]. Over the lifespan, the capsule increases in size and becomes distorted in shape due to the addition of new lamellae around the periphery [67]. The capsule acts as a high-pass filter that limits low-frequency and steadystate pressure stimuli from influencing membrane permeability of the axon terminal, with a best frequency at approximately 250 Hz [68-70]. The anatomic relation of the PC receptor to surrounding integument translates to relatively large receptive fields (several millimeters-to-centimeters in diameter), and well suited for spatial summation [71]. Encapsulated Ruffini corpuscles show directional sensitivity to skin stretch and are in the deepest layer of the dermis known as the reticular layer [1,58-61]. Using microneurography, it has been shown that two types of rapidly adapting units, RA I (Meissner c.) and RA II (Pacinian c.), respond to incipient slippage in the finger-object contact area [73].
It appears that RA I units are the predominant detectors of local slippage and are responsible for grip adjustment, whereas RA II units do not localize partial slippage during precision grip because of their large receptive fields [72]. The interaction of the hand with touched objects results in the propagation of mechanical energy throughout the hand primarily in the 10-100 Hz frequency band which in turn excites mechanoreceptors over wide areas [74,75]), including Pacinian corpuscles owing to their relatively large receptive fields and frequency sensitivity (∼20 Hz to 1 kHz) [66,76- 80]. Other mechanoreceptors such as Merkel cell neurites (SA I), respond to mechanical inputs over the tactile frequency range [81,82]). Similarly, the numerous Meissner corpuscles (RA I) found in the epidermal grooves of the glabrous skin are most responsive to low-frequency mechanical inputs (10–200 Hz) [83].
Contrasting cutaneous sensitivity of the Hand and Face: The differences in the vibrotactile sensitivities of the hand [43,70,84- 86] and face [42,43,87,88] are confirmed in the present report using the SIUD adaptive threshold protocol. The classic Pacinian U-shaped response characteristic in the glabrous hand for vibratory input at 250 Hz is virtually absent in perioral hairy skin [43,44,60,89-91]. This is consistent with histological and physiological studies of facial skin which have not found PC receptors [47,92,93]. The majority of facial muscle fibers insert directly into the skin rather than the connective tissue making it possible for embedded mechanoreceptors such as Ruffini endings to encode proprioceptive information about changes in muscle length and force [50,60].
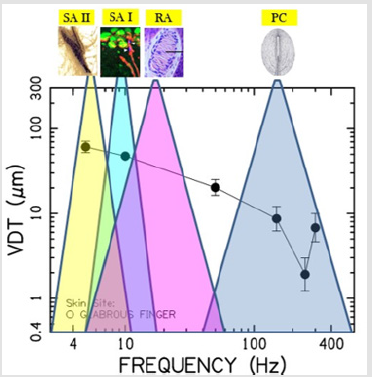
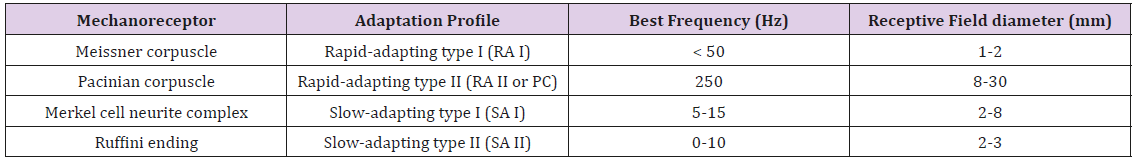
This is interesting given that muscle spindle receptors and Golgi tendon organs, common to limb systems, have not been found in the lower face [94,95]. Mechanoreceptive afferents found in the hand and face can be distinguished by their adaptation profile, best frequency, and receptive field size (Table 3). In a psychophysical vibrotactile detection paradigm such as described in the current report, it is the combination of mechanoreceptor types and their respective physiological features (adaptation profile, BF, receptive field size, surrounding integument tissue properties, primary afferent projections, functional representation in the brain, and neurological status) which ultimately determine the mechanical frequency detection threshold function for any given skin site, herein known as a vibrogram (Figure 4).
Figure 4: The vibrogram and putative mechanoreceptor typing, frequency sensitivities, and potential contribution to the glabrous hand VDT.
Vibrotactile sensing and emergent haptic technologies. Biological communication modeling and systems design through neurons and networks [96], is engaging haptics to interface brain (mind) and machine, enhance motor control, and develop artificial tactile sensing using 200 Hz probes for object-shape recognition [97] to complement auditory and visual data streams for cyberphysical information flow and smart device control.
Table 3: Classification of cutaneous mechanoreceptors based on their responses to an applied force, the frequency to which each best responds and the size of their receptive fields.
As the largest organ of our body, our skin provides tactile channels that can be used for communication in a variety of taskspecific haptic interface designs [98]. Haptic sensations offer an additional method of communication between systems and operators in environments in which there is visual or auditory overload [99,100]), including the encoding of touch force over a broad spectrum of inputs (5-1000 Hz) [101], presentation of music spanning musical notes C1 (32.7 Hz) to C6 (1046.5 Hz) as vibration to the glabrous skin to facilitate interaction between musicians with hearing impairments and other musicians during group performances [102], or in mission critical situations in which fighter jet pilot controls utilize a haptic interface that is integrated within an antigravity suit using multichannel vibrotactile inputs (varying in frequency between 27.23 Hz and 152.92 Hz) to the lower back, outer/inner thighs, and outer/inner calves [100].
Brain stimulation techniques guided by tactile sensing technologies are showing promise in neurotherapeutics and functional investigation of the brain [103-105]. For example, some investigators are exploring the effects of transcranial direct current stimulation on neuromodulation of primary somatosensory cortex on VDT detection and somatosensory discrimination [106]. Focused Ultrasound (FUS) sonication of S1 has been used to evoke both sonication- specific electroencephalographic (EEG) responses and various tactile sensations from the hand area of the postcentral gyrus [107]. Others have used mapping of local field potentials to elucidate the functional properties of cortical areas involved in multimodal processing of somatosensory inputs, such as the posterior insula, which is regarded as the so-called ‘ouch-zone’ and presumed to play a key role pain perception. Direct intracerebral recordings, however, have shown that painful and nonpainful stimuli elicit very similar responses throughout the human insula. Non-nociceptive somatosensory stimuli consisted of 250 Hz vibratory bursts (50 ms) on the palmar surface of the index fingertip [108].
Artificial sensory feedback systems is a rapidly emerging technology which incorporates small vibrating tactors, placed at different parts of the body, to provide spatial as well as temporal feedback to compensate for lost proprioception in individuals with lower-limb impairments [109]. Vibrotactile feedback has demonstrated efficacy in individuals with lower-limb amputations, vestibular impairments, and aging-related loss of balance [110-114]. The targeted vibrations range from 60-400 Hz to match the spectral response profiles of cutaneous mechanoreceptors [110,115,116]. Tracking changes in VDT sensitivity is being used to develop new therapeutic approaches to enhance plantar sensitivity to minimize postural instability in progressive movement disorders such as Parkinson’s disease [115]. The modulation of precision grip by anticipatory vibrotaction during 100 Hz and 250 Hz stimulation provides important evidence on the role of mechanoreception to influence motor reflex action during voluntary movement [118]. VDTs obtained using 125 Hz stimuli have been used to follow the progression of peripheral neuropathy in the digits [119].
Conclusion
Our study demonstrated that an automated SIUD adaptive threshold tracking procedure can reliably assess vibrotactile sensitivity for the hand and face in neurotypical adults in a timely manner. This SIUD procedure replicated previous findings which have shown significant main effects for stimulation site and stimulus frequency presumably due to the differences in the density and type of mechanoreceptors innervating the face and glabrous hand. Vibrotactile testing can be used noninvasively map the integrity of Aβ somatosensory pathways. In our present implementation of the automatic SIUD method, the VDT function at a single skin site based on 6 test frequencies (5, 10, 50, 150, 250, 300 Hz) can be measured in approximately 3 minutes 45 seconds. The range of stimulus test frequencies is user-defined and can be expanded to include 400 and 600 Hz vibratory inputs as well.
The incidence of brain injury and progressive neurological disease increases with age, and the sense of touch is altered in older adults [48,120,121]. Automated VDT testing can be used to determine the extent of sensory impairment, and through repeated measurements monitor the progress of the disease or injury, and treatment efficacy.
Acknowledgment
Special gratitude is expressed to Jari Janis Billiot, Katie Beth Hundley, Chelsey Krug, Kelsey Sestak, AnnaJean Scarborough, and Doug Kieweg for assistance with data collection in this study.
Disclosure Statement
The authors alone are responsible for the content and writing of the paper. The authors have no conflicts of interest or financial disclosures relevant to this work.
Funding
This work was supported, in part, by the Barkley Trust Foundation at the University of Nebraska-Lincoln (Barlow).
References
- Arda O, Göksügür N, Tüzün Y (2014) Basic histological structure and functions of facial skin. Clinics in Dermatology 32(1): 3-13.
- Carey L, Lamp G, Turville M (2016) The state-of-the-science on somatosensory function and its impact on daily life in adults and older adults and following stroke: a scoping review. OTJR: Occupation, Participation and Health 36(25): 275-415.
- Kim J (2007) Patterns of sensory abnormality in cortical stroke: evidence for a dichotomized sensory system. Neurology 68(3): 174-180.
- Mah Y, Husain M, Rees G, Nachev P (2014) Human brain lesion-deficit inference remapped. Brain 137(Pt 9): 2522-2531.
- Meyer S, De Bruyn N, Lafosse C, Van Dijk M, Michielsen M, et al. (2016a) Somatosensory impairments in the upper limb poststroke: distribution and association with motor function and visuospatial neglect. Neurorehabilitation and Neural Repair 30(8): 731-742.
- Kenzie J, Semrau J, Findlater S, Yu A, Desai J, et al. (2016) Localization of impaired kinesthetic processing in post-stroke. Frontiers in Human Neuroscience 10(505): 1-13.
- Nijboer T van de port I, Schepers V, Post M, Visser Meily A (2013) Predicting functional outcome after stroke: the influence of neglect on basic activities in daily living. Frontiers in Human Neuroscience 7(182): 1-6.
- Torre K, Hammami N, Metrot J, van Dokkum L, Coroian F, et al. (2013) Somatosensory- related limitation for bimanual coordination after stroke. Neurorehabilitative Neural Repair 27(6): 507-515.
- Meyer S, Kessner S, Cheng B, Bönstrup M, Schulz R, Hummel F (2016b) Voxel-based lesion-symptom mapping of stroke lesions underlying somatosensory deficits. NeuroImage Clinical 10: 257-266.
- Marmarou A, Anderson R, Ward J, Sung C Choi, Harold F Young, et al. (1991) Impact of ICP instability and hypotension on outcome in patients with severe head trauma. Journal of Neurosurgery 75: S59-66.
- Webster K, Sun M, Crack P, O Brien T, Shultz S, et al. (2017) Inflammation in epileptogenesis after traumatic brain injury. Journal of Neuroinflammation 14(10): 1-17.
- Mendez C, Hurley R, Lassonde M, Zhang L, Taber K (2005) Mild traumatic brain injury: neuroimaging of sports-related concussion. Journal of Neuropsychiatry Clinical Neuroscience 17(3): 297-303.
- Beaumont A, Marmarou A, Czigner A, Yamamoto M, Demetriadou K, et al. (1999) The impact-acceleration model of head injury: Injury severity predicts motor and cognitive performance after trauma. Neurology Research 21(8): 742-754.
- De Beaumont L, Lassonde M, Leclerc S, Theoret H (2007) Long-term and cumulative effects of sports concussions on motor cortex inhibition. Neurosurgery 61(2): 329-337.
- Laskowski R, Creed J, Raghupathi R (2015) Pathophysiology of mild TBI: implications for altered signaling pathways. In: Kobeissy FH, editor. Brain Neurotrauma: Molecular, Neuropsychological, and Rehabilitation Aspects. Boca Raton (FL): CRC Press/Taylor & Francis.
- Schneider J, Diamond S, Markham C (1986) Deficits in orofacial sensorimotor function in Parkinson's disease. Annals of Neurology 19(3): 275-282.
- Shin H, Kang S, Sohn, Y (2005) Dopaminergic influence on disturbed spatial discrimination in Parkinson’s disease. Movement Disorders 20(12): 1640-1643.
- Cao H, Xu X, Zhao Y, Long D, Zhang M (2011) Altered brain activation and connectivity in early Parkinson disease tactile perception. American Journal of Neuroradiology 32(10): 1969-1974.
- Conte A, Khan N, Defazio G, Rothwell J, Berardelli A (2013) Pathophysiology of somatosensory abnormalities in Parkinson disease. Nature Reviews Neurology 9(12): 687-697.
- Lee M, Lyoo C, Lee M, Sim J, Cho H, Choi Y (2010) Impaired finger dexterity in patients with Parkinson's disease correlates with discriminative cutaneous sensory dysfunction. Movement Disorders 25(15): 2531-2535.
- Nelson A, Premji A, Rai N, Hoque T, Tommerdahl M, Chen R (2012) Dopamine alters tactile perception in Parkinson's disease. The Canadian Journal of Neurological Science 39(1): 52-57.
- Brefel Courbon C, Payoux P, Thalamas C, Ory F, Quelven I, et al. (2005) Effect of levodopa on pain threshold in Parkinson's disease: a clinical and positron emission tomography study. Movement Disorders 20(12): 1557-1563.
- Ciampi de Andrade D, Lefaucheur J, Galhardoni R, Ferreira K, Brandão Paiva A, et al. (2012) Subthalamic deep brain stimulation modulates small fiber-dependent sensory thresholds in Parkinson's disease. Pain 153 (5): 1107-1113.
- Kawamura Y, Dyck P, Shimono M (1981) Morphometric comparison of the vulnerability of peripheral motor and sensory neurons in amyotrophic lateral sclerosis. Journal of Neuropathology Experimental Neurology 40(6): 667-675.
- Guo Y, Wu D, Wu H, Wu SY, Yang C, et al. (2009) Sensory involvement in the SOD1-G93A mouse model of amyotrophic lateral sclerosis. Experimental Molecular Medicine 41(3): 140-150.
- Sábado J, Casanovas A, Tarabal O, Hereu M, Piedrafita L, et al. (2014) Accumulation of misfolded SOD1 in dorsal root ganglion degenerating proprioceptive sensory neurons of transgenic mice with amyotrophic lateral sclerosis. BioMed Research International 2014: ID 852163: 1-13.
- Iglesias C, Sangari S, El Mendili M, Benali H, Marchand Pauvert V, et al. (2015) Electrophysiological and spinal imaging evidences for sensory dysfunction in amyotrophic lateral sclerosis. BMJ Open 5(2): e007659.
- Cohen Adad J, El Mendili M, Morizot Koutlidis R, Lehéricy S, Meininger V, et al. (2013) Involvement of spinal sensory pathway in ALS and specificity of cord atrophy to lower motor neuron degeneration. Amyotrophic Lateral Sclerosis and Frontotemporal Degeneration 14(1): 30-38.
- Hammad M, Silva A, Glass J, Sladky JT, Benatar M (2007) Clinical, electrophysiologic, and pathologic evidence for sensory abnormalities in ALS. Neurology 69(24): 2236-2242.
- Tomešová J, Gruberova J, Lacigova S, Cechurova D, Jankovec Z, et al. (2013) Differences in skin microcirculation on the upper and lower extremities in patients with diabetes mellitus: relationship of diabetic neuropathy and skin microcirculation. Diabetes Technology & Therapeutics 15(11): 968-975.
- Burakgazi A, AlMahameed S (2016) Cardiac Involvement in Peripheral Neuropathies. Journal of Clinical Neuromuscular Disease 17(3): 120-128.
- Joo Kim Y, Rogers J, Kwok G, Dunn W, Holm M (2016) Somatosensation Differences in Older Adults with and Without Diabetes, and by Age Group. Occupational Therapy in Healthcare 30(3): 231-244.
- Tsai H, Sung Y, de Jesus Perez V (2016) Recent advances in the management of pulmonary arterial hypertension. F1000 Research 5(2755): 1-10.
- Lincoln N, Crow J, Jackson J, Waters G, Adams S, Hodgson P (1991) The unreliability of sensory assessment. Clinical Rehabilitation 5: 273-282.
- Smania N, Montagnana B, Faccioli S, Fiaschi A, Aglioti S (2003) Rehabilitation of somatic sensation and related deficit of motor control in patients with pure sensory stroke. Arch Phys Med Rehabilitation 84(11): 1692-1702.
- Connell L, Lincoln N, Radford K (2008) Somatosensory impairment after stroke: frequency of different deficits and their recovery. Clinical Rehabilitation 22(8): 758-767.
- Winward C, Halligan P, Wade D (1999) Current practice and clinical relevance of somatosensory assessment after stroke. Clinical Rehabilitation 13(1): 48-55.
- Sommerfield D, von Arbin M (2004) The impact of somatosensory function on activity performance and length of hospital stay in geriatric patients with stroke. Clinical Rehabilitation 18(2): 149-155.
- Gray D, Hollingsworth H, Stark S, Morgan K (2006) Participation survey/mobility: Psychometric properties of a measure of participation for people with mobility impairments and limitations. Archives of Physical Medicine and Rehabilitation 87(2): 189-197.
- Baum C (2011) Fulfilling the promise: Supporting participation in daily life. Archives of Physical Medicine and Rehabilitation 92(2): 169-175.
- Lecluyse W, Meddis R (2009) A simple single-interval adaptive procedure for estimating thresholds in normal and impaired listeners. The Journal of the Acoustical Society of America 126(5): 2570-2579.
- Venkatesan L, Barlow SM, Kieweg D (2015) Age- and sex-related changes in vibrotactile sensitivity of hand and face in neurotypical adults. Somatosensory Motor Research 32(1): 44-50.
- Barlow SM (1987) Mechanical frequency detection thresholds in the human face. Experimental Neurology 96(2): 253-261.
- Johansson R, Trulsson M, Olsson K, Westberg K (1988) Mechanoreceptor activity from the human face and oral mucosa. Experimental Brain Research 72(1): 204-208.
- Trulsson M, Johansson R (2002) Orofacial mechanoreceptors in humans: encoding characteristics and responses during natural orofacial behaviors. Behavioral Brain Research 135(1-2): 27-33.
- Hollins M, Delemos K, Goble A (1991) Vibrotactile adaptation on the face. Perception & Psychophysics 49(1): 21-30.
- Munger B, Halata Z (1983) The sensory innervation of primate facial skin. I. Hairy skin. Brain Research 286(1): 45-80.
- Gescheider G, Bolanowski S, Hall K, Hoffman K, Verrillo R (1994) The effects of aging on information-processing channels in the sense of touch: I. Absolute sensitivity. Somatosensory & Motor Research 11(4): 345-357.
- Verrillo R (1979a) Comparison of vibrotactile threshold and suprathreshold responses in men and women. Perception & Psychophysics 26(1): 20-24.
- Barlow SM (1998) Real time modulation of speech--orofacial motor performance by means of motion sense. Journal of Communication Disorders 31(6): 511-533.
- Merkel FS (1875) Tastzellen und tastkörperchen bei den hausthieren und beim menschen. Archiv für mikroskopische Anatomie 11: 636-652.
- Hashimoto K (1972) The ultrastructure of the skin of human embryos. X. Merkel tactile cells in the finger and nail. Journal of Anatomy 111(1): 99-120.
- Briggaman R, Wheeler C (1975) The epidermal-dermal junction. Journal of Investigative Dermatology 65(1): 71-84.
- Corcuff P, Bertrand C, Leveque J (1993) Morphometry of human epidermis in vivo by real-time confocal microscopy. Archives of Dermatology Research 285: 475-481.
- Halata Z, Grim M, Bauman K (2003) Friedrich Sigmund Merkel and his "Merkel cell", morphology, development, and physiology: review and new results. The Anatomical Record. Part A. Discoveries in Molecular, Cellular and Evolutionary Biology 271(1): 225-239.
- Morrison K, Miesegaes G, Lumpkin E, Maricich S (2009) Mammalian merkel cells are descended from the epidermal lineage. Developmental Biology 336(1): 76-83.
- Young B, Lowe J, Stevens A, Heath J (2006) Wheater’s Functional Histology: A Text and Colour Atlas. 5th ed. London: Churchill Livingstone, pp. 167-185.
- Mescher A (2010) Junqueira’s Basic Histology: Text and Atlas. (12th). Singapore: McGraw-Hill Medical pp. 480.
- Kierszenbaum A, Tres L (2012) Histology and Cell Biology: An Introduction to Pathology. 3rd Philadelphia: Elsevier Saunders, pp. 339-363.
- Nordin M, Hagbarth K (1989) Mechanoreceptive units in the human infra-orbital nerve. Acta Physiologica Scandinavica 135(2): 149-161.
- Pawlina W, Ross M (2011) Histology: A Text and Atlas. (6th). Philadelphia: Lippincott Williams & Wilkins, pp 488-525.
- Trulsson M, Essick G Mechanosensation (2004) In: Miles TS, Nauntofte B, Svensson P (Eds.), Clinical Oral Physiology. Copenhagen: Quintessence Books, pp. 165-197.
- Toda T, Taoka M (2004) Converging patterns of inputs from oral structures in the postcentral somatosensory cortex of conscious macaque monkeys. Experimental Brain Research 158(1): 43-49.
- Rosner A, Barlow SM (2016) Hemodynamic changes in cortical sensorimotor systems following hand and orofacial motor tasks and pulsed pneumotactile stimulation. Somatosensory & Motor Research 33(3-4): 145-155.
- Morrison K, Miesegaes G, Lumpkin E, Maricich S (2009) Mammalian merkel cells are descended from the epidermal lineage. Developmental Biology 336(1): 76-83.
- Cauna N, Mannan G (1958) The structure of human digital Pacinian corpuscles (corpus cula lamellosa) and its functional significance. Journal of Anatomy 92(1): 1-20.
- Cauna N (1965) The effects of aging on the receptor’s organs of the human dermis. In: Montagna W, editor. Advances in Biology of Skin, New York, NY: Pergamon Press, p. 63-96.
- Loewenstein W, Mendelson M (1965) Components of receptor adaptation in a Pacinian corpuscle. Journal of Physiology 177: 377-397.
- Loewenstein W, Skalak R (1966) Mechanical transmission in a Pacinian corpuscle. An analysis and a theory. Journal of Physiology 182(2): 346-378.
- Verrillo R (1979b) Change in vibrotactile thresholds as a function of age. Sensory Processes 3(1): 49-59.
- Gescheider G, Bolanowski S, Verrillo R (2004) Some characteristics of tactile channels. Behavioral Brain Research 148(1-2): 35-40.
- Johnson K (2001) The roles and functions of cutaneous mechanoreceptors. Current Opinions in Neurobiology 11(4): 455-461.
- Johansson R, Westling G (1987) Signals in tactile afferents from the fingers eliciting adaptive motor responses during the precision grip. Experimental Brain Research 66(1): 141-154.
- Johansson R, Flanagan J (2009) Coding and use of tactile signals from the fingertips in object manipulation tasks. Nature Reviews Neuroscience 10(5): 345-359.
- Shao Y, Hayward V, Visell Y (2016) Spatial patterns of cutaneous vibration during whole-hand haptic interactions. PNAS 113(15): 4188-4193.
- Johansson R, Vallbo A (1979) Tactile sensibility in the human hand: Relative and absolute densities of four types of mechanoreceptive units in glabrous skin. Journal of Physiology 286: 283-300.
- Järvilehto T, Hämäläinen H, Soininen K (1981) Peripheral neural basis of tactile sensations in man: II. Characteristics of human mechanoreceptors in the hairy skin and correlations of their activity with tactile sensations. Brain Research 219(1): 13-27.
- Kumamoto K, Senuma H, Ebara S, Matsuura T (1993) Distribution of Pacinian corpuscles in the hand of the monkey, Macaca fuscata. Journal of Anatomy 183(1): 149-154.
- Stark B, Carlstedt T, Hallin R, Risling M (1998) Distribution of human Pacinian corpuscule in the hand. Journal of Hand Surgery 23(3): 370-372.
- Edin B, Abbs J (1991) Finger movement responses of cutaneous mechanoreceptors in the dorsal skin of the human hand. Journal of Neurophysiology 65(3): 657-670.
- Gottschaldt K, Vahle Hinz C (1981) Merkel cell receptors: Structure and transducer function. Science 214(4517): 183-186.
- Maksimovic S, Nakatani M, Baba Y, Nelson A, Marshall K, et al. (2014) Epidermal Merkel cells are mechanosensory cells that tune mammalian touch receptors. Nature 509(7502): 617-621.
- Goodwin A, Youl B, Zimmerman N (1981) Single quickly adapting mechanoreceptive afferents innervating monkey glabrous skin: Response to two vibrating probes. Journal of Neurophysiology 45(2): 227-242.
- Verrillo R (1980) Age related changes in the sensitivity to vibration. Journal of Gerontology 35(2): 185-193.
- Verrillo R (1982) Effects of aging on the suprathreshold responses to vibration. Perception & Psychophysics 32(1): 61-68.
- Verrillo R (1983) Vibrotactile subjective magnitude as a function of hand preference. Neuropsychologia 21(4): 383-395.
- Verrillo R, Ecker A (1977) Effects of root or nerve destruction on vibrotactile sensitivity in trigeminal neuralgia. Pain 3(3): 239-255.
- Andreatta R, Davidow J (2006) Mechanical frequency and stimulation-site-related differences in vibrotactile detection capacity along the lip vermilion in young adults. Clinical Oral Investigations 10(1): 17-22.
- Kent R, Martin R, Sufit R (1990) Oral sensation: A review and clinical prospective. In H. Winitz (Ed.), Human Communication and its Disorders, Norwood, NJ: Ablex Press.
- Andreatta RD, Davidow JH, Scott AT (2003) Low-level static lip force control does not alter vibrotactile detection thresholds in the human orofacial system. Exp Brain Res 151(4): 548-552.
- Andreatta RD, Barlow SM (2003) Movement-related modulation of vibrotactile detection thresholds in the human orofacial system. Exp Brain Res 149(1): 75-82.
- Dubner R, Sessle B, Storey A (1978) The neural basis of oral and facial function. New York: Plenum.
- Halata Z, Munger B (1983) The sensory innervation of primate facial skin. II. Vermilion border and mucosal lip. Brain Research 286(1): 81-107.
- Stal P, Eriksson P, Eriksson A, Thornell L (1987) Enzyme-histochemical differences in fibretype between the human major and minor zygomatic and the first dorsal interosseus muscles. Archives of Oral Biology 32(11): 833-841.
- Stal P, Eriksson P, Eriksson A, Thornell L (1990) Enzyme-histochemical and morphological characteristics of muscle fibre types in the human buccinator and orbicularis oris. Archives of Oral Biology 35(6): 449-458.
- Akyildiz I, Pierobon M, Balasubramaniam S, Koucheryavy Y (2015) The internet of bionano Things. IEEE Communications Magazine 53(3): 32-40.
- Khasnobish A, Pal M, Sardar D, Tibarewala D (2016) Vibrotactile feedback for conveying object shape information as perceived by artificial sensing of robotic arm. Cognitive Neurodynamics 10(4): 327-338.
- Montagu, A (1986) Touching: the Human Significance of the Skin, (3rd edn). Harper and Row, New York.
- Spence C, Ngo M, Lee J, Tan H (2010) Solving the correspondence problem in haptic/multisensory interface design. Advances in Haptics 1: 47-74.
- Ko S, Lee K, Kim D, Ji Y (2017) Vibrotactile perception assessment for a haptic interface on an antigravity suit. Applied Ergonomics 58: 198-207.
- Hatzfeld C, Cao S, Kupnik M, Werthschützky R (2016) Vibrotactile Force Perception - Absolute and Differential Thresholds and External Influences. IEEE Transactions on Haptics 9(4): 586-597.
- Hopkins C, Maté Cid S, Fulford R, Seiffert G, Ginsborg J (2016) Vibrotactile presentation of musical notes to the glabrous skin for adults with normal hearing or a hearing impairment: thresholds, dynamic range and high-frequency perception. PLoS ONE 11(5): e0155807.
- George M, Aston Jones G (2010) Noninvasive techniques for probing neurocircuitry and treating illness: vagus nerve stimulation (VNS), transcranial magnetic stimulation (TMS) and transcranial direct current stimulation (tDCS). Neuropsychopharmacology 35(1): 301-316.
- Hoy K, Fitzgerald P (2010) Brain stimulation in psychiatry and its effects on cognition. Nature Reviews Neurology 6(5): 267-275.
- Lee W, Chung Y, Jung Y, Song I, Yoo S (2016) Simultaneous acoustic stimulation of human primary and secondary somatosensory cortices using transcranial focused ultrasound. BMC Neuroscience 17(68): 1-11.
- Labbé S, Meftah E, Chapman C (2016) Effects of transcranial direct current stimulation of primary somatosensory cortex on vibrotactile detection and discrimination. Journal of Neurophysiology 115(4): 1978-1987.
- Lee W, Kim H, Jung Y, Song I, Chung Y, Yoo S (2015) Image-guided transcranial focused ultrasound stimulates human primary somatosensory cortex. Scientific Reports 5: 8743.
- Liberati G, Klöcker A, Safronova MM, Santos SF, Vaz JG, et al. (2016) Nociceptive local field potentials recorded from the human insula are not specific for nociception. PLoS Biology 14(1): e1002345.
- Leineweber M, Shi S, Andrysek J (2016) A method for evaluating timeliness and accuracy of volitional motor responses to vibrotactile stimuli. Journal of Visualized Experiments (114): e54223.
- Wentink E, Mulder A, Rietman J, Veltink P (2011) Vibrotactile stimulation of the upper leg: Effects of location, stimulation method and habituation. In Engineering in Medicine and Biology Society, EMBC, Annual International Conference of the IEEE 2011(Aug 30): 1668-1671.
- Rusaw D, Hagberg K, Nolan L, Ramstrand N (2012) Can vibratory feedback be used to improve postural stability in persons with transtibial limb loss? Journal of Rehabilitation Research & Development 49(8): 1239-1254.
- Goodworth A, Wall C, Peterka R (2009) Influence of feedback parameters on performance of a vibrotactile balance prosthesis. IEEE Translational Neural Systems in Rehabilitation 17(4): 397-408.
- Asseman F, Bronstein A, Gresty M (2007) Using vibrotactile feedback of instability to trigger a forward compensatory stepping response. Journal of Neurology 254(11): 1555-1561.
- Fan R, Culjat M, King C, Franco M, Boryk R, et al. (2008) A haptic feedback system for lower-limb prostheses. IEEE Translational Neural Systems in Rehabilitation 16(3): 270-277.
- Bolanowski S, Gescheider G, Verrillo R, Checkosky C (1988) Four channels mediate the mechanical aspects of touch. Journal of the Acoustical Society of America 84(5): 1680-1694.
- Sharma A, Torres Moreno R, Zabjek K, Andrysek J (2014) Toward an artificial sensory feedback system for prosthetic mobility rehabilitation: Examination of sensorimotor responses. Journal of Rehabilitation. Research & Development 51(6): 416-425.
- McKeown M, Peters R, Pasman E, McKeown M, Carpenter M, et al. (2016) Plantar cutaneous function in Parkinson’s disease patients ON and OFF L-dopa. Neuroscience Letters 629: 251-255.
- Okamoto S, Wiertlewski M, Hayward V (2016) Anticipatory Vibrotactile Cueing Facilitates Grip Force Adjustment during Perturbative Loading. IEEE Transactions on Haptics 9(2): 233-242.
- Takemura S, Yoshimasu K, Tsuno K, Fukumoto J, Kuroda M, et al. (2016) Associations between anthropometric factors and peripheral neuropathy defined by vibrotactile perception threshold among industrial vibrating tool operators in Japan. Journal of Occupational Health 58: 145-154.
- Stevens J, Choo K (1996) Spatial acuity of the body surface over the life span. Somatosensory & Motor Research 13(2): 153-166.
- Stevens J, Cruz L (1996) Spatial acuity of touch: ubiquitous decline with aging revealed by repeated threshold testing. Somatosensory & Motor Research 13(1): 1-10.

 Research Article
Research Article