Abstract
Objective: Temporal lobectomy is often successful in treatment of patients with medically intractable temporal lobe epilepsy (TLE), but prediction of its long-term outcome is of great interest. Here, we investigated usefulness of comparison analysis of pre- and post-surgical magnetoencephalopgrahy data in predicting prognosis of temporal lobectomy.
Methods: The authors retrospectively analyzed pre-and post-operative MEG and presence of any seizures after temporal lobectomy in 8 patients with TLE. Spectral powers averaged from spike-free epochs in each condition (pre- and post-operation) were compared between 4 patients free from seizures and the other 4 with recurrent seizures. We also performed connectivity analysis based on phase locking values (PLVs) around resection margins of possible epileptogenic focus as regions of interest.
Results: Spectral analysis on peri-lesional areas demonstrated relative increase of delta power in the patients without seizure freedom. On connectivity analysis, decrease of PLVs between other areas and temporal lobe focus after surgery were noted in patients with good surgical outcome while persistence or increase were found in patients with recurred seizures.
Conclusion: This pilot study on comparison of power spectrum and connectivity metrics between pre- and post-op MEG exhibits a potential for predicting seizure outcome after TLE surgery.
Keywords: Epilepsy; Magnetoencephalography; Prognosis; Temporal lobectomy; Connectivity
Introduction
For patients with medically intractable temporal lobe epilepsy (TLE), anterior temporal resection has been mostly frequent treatment. However, there is a substantial variability in success rate of the surgery. Studies examining 1-year outcomes of TLE showed seizure free rate is largely 60 – 80% [1,2]. Prediction of success after TLE surgery for each patient is often of a great issue and several studies addressed it. Clinical and neuroimaging factors predicting favorable outcome are focal findings of mesial temporal sclerosis seen either neuroimaging and pathologic finding, unilateral and less extensive hypometabolism on positron emission tomography (PET), neuropsychological findings suggestive of unilateral dysfunction ipsilateral to probable epileptogenic side, shorter duration, and absence of generalized tonic-clonic seizure history [3,4]. Unlike functional magnetic resonance imaging, electrophysiologic studies such as electroencephalography (EEG) and magnetoenecephalography(MEG)[1,4] techniques directly measure local field potential of electric brain activity [5].
Despite of evidence that scalp EEG can be useful in predicting seizure outcome in epilepsy surgery, its utility is still uncertain due to limitation concerning low (up to 1 cm) spatial resolution and signal distortion by skull and scalp [6]. On the other hand, MEG has a few advantages over EEG in mapping epileptogenic focus owing to higher spatial resolution and sensitivity to tangential dipole. Therefore, theoretically, epilepsy surgery based on MEG and has substantial benefits compared to EEG. Recent reports support this concept that regional spikes and their clusters evaluated by dipole localization based on MEG data is found to be highly accurate in localizing epileptic source and prediction of outcome [7,8]. Another method, phase synchronization which can be calculated by resting state dynamics based on correlation of pairs of brain signal of each area in whole brain is used to elucidate abnormal synchrony of epileptic brain [9]. Abnormal hypersynchrony of seizure generating areas and adjacent brain regions was found in intracranial EEG, and moreover, surgical removal of synchronized clusters was correlated with good seizure outcome [10].
Also, MEG recordings with TLE patients revealed altered functional network [11,12] and a few studies showed its feasibility for prediction of postsurgical outcome [13,14]. But to date, there has been few studies which demonstrates usefulness of longitudinal analysis of both pre- and post-operative MEG recording for predicting seizure outcome [13]. Our main aim was to find neurophysiologic biomarkers that can help predict seizure outcome after temporal lobectomy using data acquired in two phases. In this study we used a 152 channel MEG to investigate local dynamics, especially spectral power and synchronization characteristics using both pre- and immediate post-surgical MEG data.
Materials and Methods
Subjects
This study included 8 patients who had undergone temporal lobectomy for TLE and pre- and post-op MEG recordings between 2012 and 2014. Each subject had focal seizures originating from the medial temporal lobe and refractory to antiepileptic drug medication. Pre-surgical evaluation included continuous video- EEG monitoring which revealed a presumed ictal onset zone in the unilateral medial temporal lobe, high resolution brain magnetic resonance imaging (MRI), radiolabeled fluorodeoxyglucose positron emission tomography (FDG PET) scan and detailed neuropsychology testing. Anatomic classification of epileptogenic foci was determined by the concordance of neurophysiologic and neuroimaging data, and ictal semiology after a comprehensive review of medical records, MRI and video-EEG monitoring. Detailed information of each patient is provided in (Table 1). We investigated duration of illness, total number of seizures, presence of GTCS, the state of antiepileptic drugs treatment until the surgery. The seizure outcome of those who were followed up for 2 years or more was assessed with presence of any seizures over 1 year at their last visit (Engel I and the others).
MEG Data Acquisition and Preprocessing
All experiments were conducted with MEG in a magnetically and electrically shielded room developed by Korea Research Institute of Standards and Science in South Korea (152 channels axial gradiometer, sampling rate: 1 kHz, notch filtering at 60 Hz and bandpass filtering with 0.1–100 Hz), simultaneous EEG (19 channels electrodes, sampling rate: 512 Hz) and Brain Products EEG (19 channels, sampling rate: 500 Hz, notch filtering at 60 Hz) systems. Two EOG channel and one EKG channel were used to exclude ocular and cardiac artifacts. In five minute-resting recordings in each MEG session, the subjects sat in a quiet room with their eyes closed.
MEG Resting State Data Analysis
4 bad channels due to mechanical problems were removed, and then a notch filter (60 Hz, 120 Hz, 180 Hz) was applied. Once filtered, the MEG data distinguished noise caused by muscle motion that is not associated brain activity like eye blinking. Also, segments including epileptiform discharges were excluded by a neurologist who is 4-year experienced EEG reviewer. The data entering into analysis was composed of more than about 150s of MEG data consisting of a noise-free eye-closed MEG segments about 1024ms. At the same time, MR images are used for cortex modeling of each subject. This procedure was performed using Freesurfer software [2] and made before and after surgery. This is to make a cortex source-based MEG. In this case, the calculated cortex ROIs are 148 in total, which is based on the destrieux model. Filtered clean MEG segments and individual subject cortex models are combined to re-calculate 148 sources-based MEG. Power-spectrum analysis and connectivity analysis were performed with the preprocessing resting state MEG. To investigate concerning peri-lesional spectrum and connectivity, 6 ROIs exhibiting greatest source localization of interictal spike visible on concomitant EEG were selected and subsequently analyzed.
Power-Spectrum Analysis: In order to compare the preoperative and postoperative results of 74 ROIs in each of the left and right hemispheres, we compared the resting state power spectrum of 6 ROIs centered on the lesion region. The ROIs were ordered according to proximity to the lesion. At this time, the MEG frequency range is 2-90Hz but divided into frequency bands. (Delta[2-4Hz], Theta[5-7Hz], Alpha[8-12Hz], Beta[15-29Hz], Lowgamma[ 30-59Hz] and High-gamma[0-90Hz]) Relative power of each band was calculated and their changes of pre- and post-op data were obtained. The averaged relative power values of each frequency were compared between the two groups subsequently.
Connectivity Analysis: Phase locking value (PLV) were used to analyze resting MEG connectivity. PLV is a statistic that can be used to investigate task-induced changes in long range synchronization of neural activity from MEG data. This method is introduced in Lachaux et al. [15]. According to the calculation method of PLV introduced in the above paper, this PLV has a value within the range of 0 to 1. This value indicated that the size of the connectivity between the 2 ROIs (Region of interest) is the closer to 1, means the higher the connectivity and a PLV close to 0 is the opposite. PLV map of each condition was thresholded by a value of 0.6. All of analyses described above were performed in brainstorm software.
Statistical Analysis: Mann-Whitney U test was used to compare spectral power between Engel Ia-outcome group (excellent outcome) and the other class group (less favorable outcome). For connectivity analysis, number of PLV based connection over the predefined threshold was compared between the two groups. This number was again tested by Mann-Whitney U test.
Results
Table 1 gives clinical characteristics, surgical and neuroimaging pathology and EEG localization of the patients. The mean age was 39.9 years (range 25 – 56; seizure-free group, 43.5; post-operative seizure group, 36.3, respectively). After surgery, patients were followed up for an average of 40.4 months. Post-op MRI revealed no visible remaining lesions.
Spectral Power Analysis
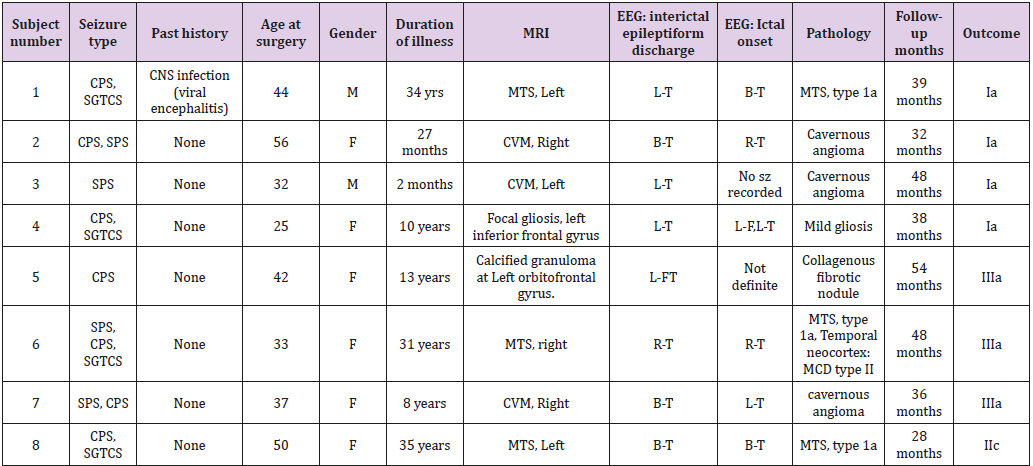
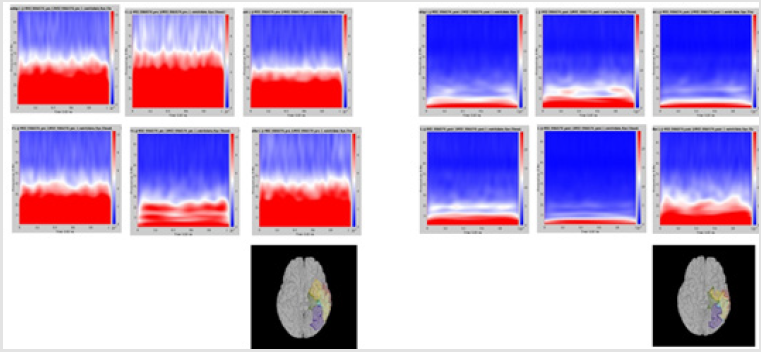
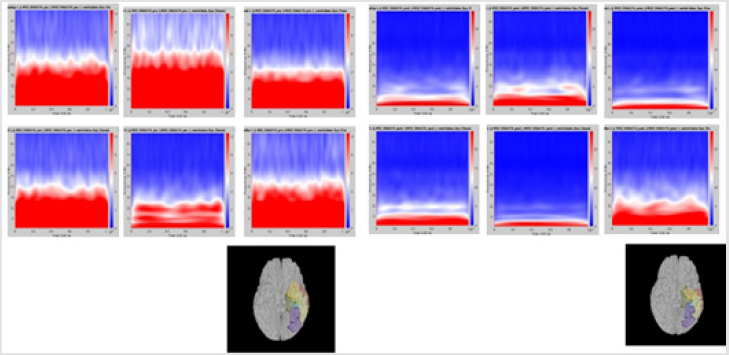
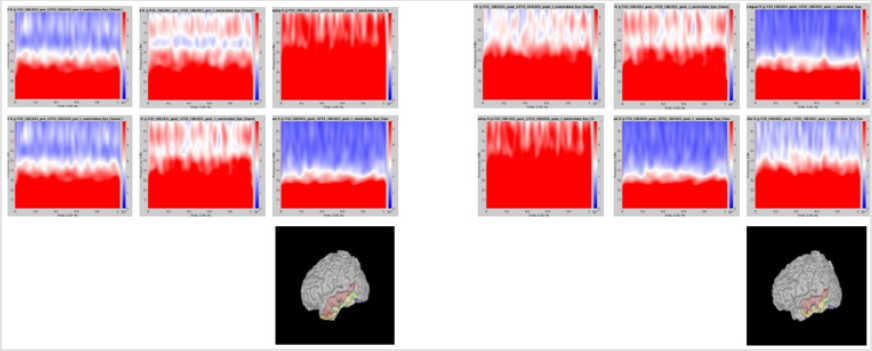
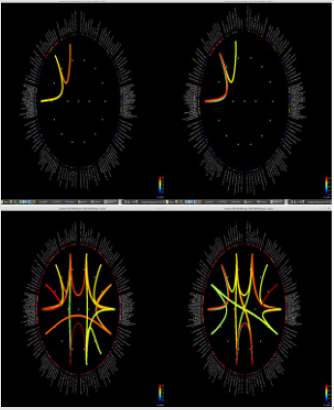
Although it did not reach statistical significance, a tendency (p = 0.056) for increase of delta frequency power after the surgery in ROI 6 of patients with persistent seizures compared to seizure-free patients was noted. Normalized spectral powers for delta frequency band on each ROIs of pre- and post-op conditions were demonstrated in (Figure 1). There was no significant difference in change of spectral powers in other frequency bands between the two groups.
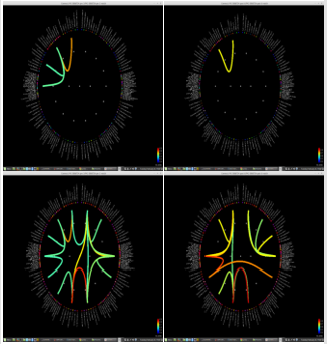
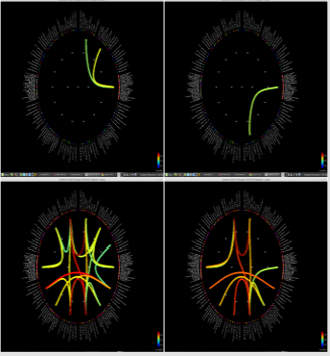
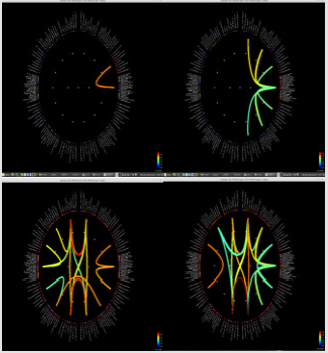
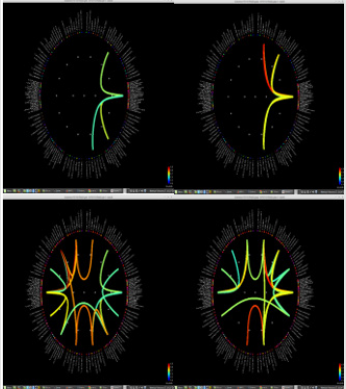
Figure 1: Delta power (compressed to 1 second time) of each ROI. Left group is the measures calculated from pre-op MEG, and right group is from pre-op MEG data. In each end of figures, each ROI is demonstrated on MRI.
Functional Connectivity based on PLV
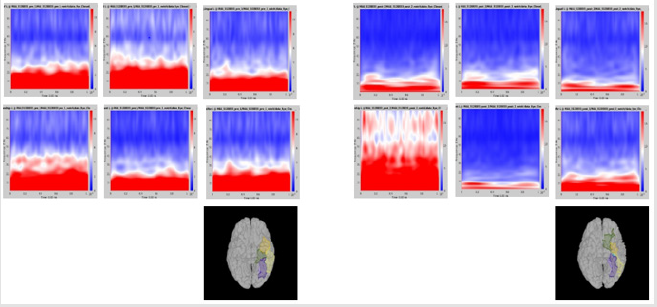
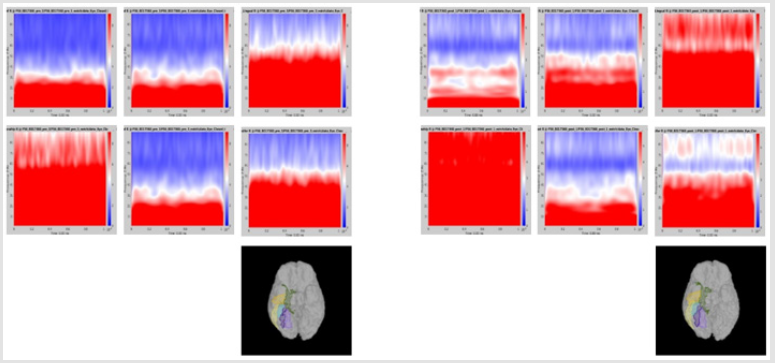
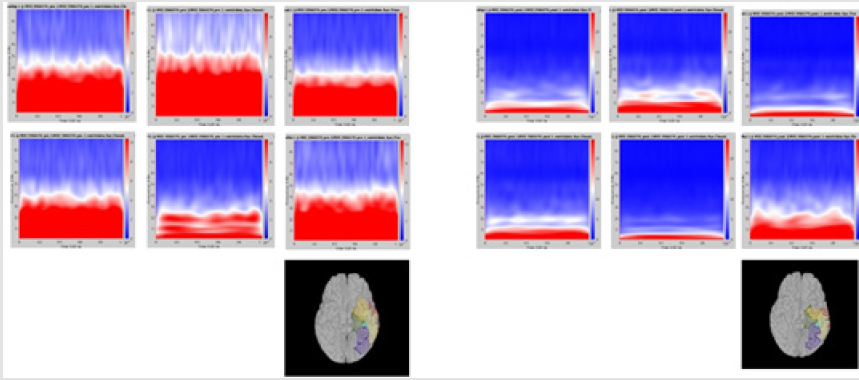
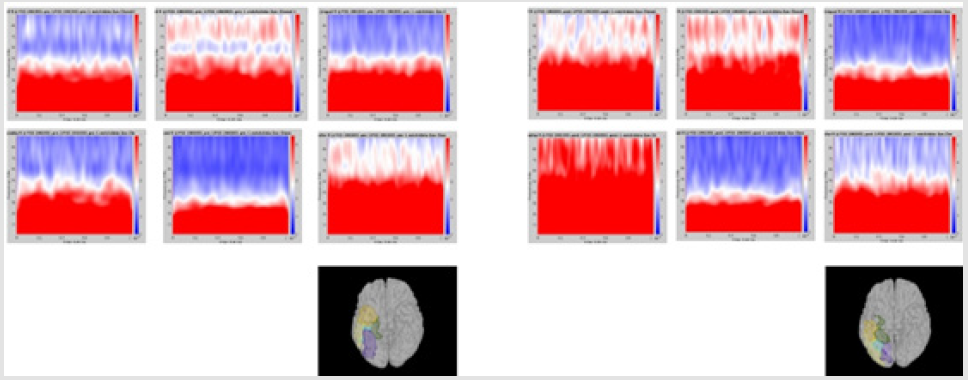
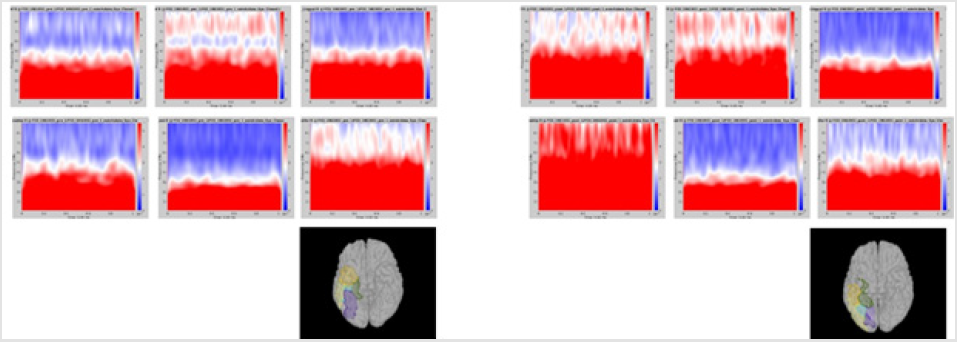
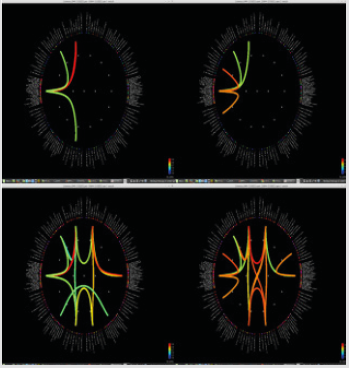
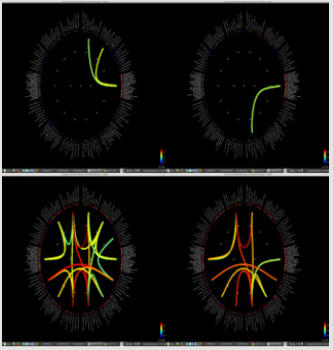
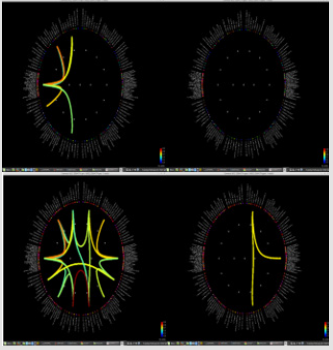
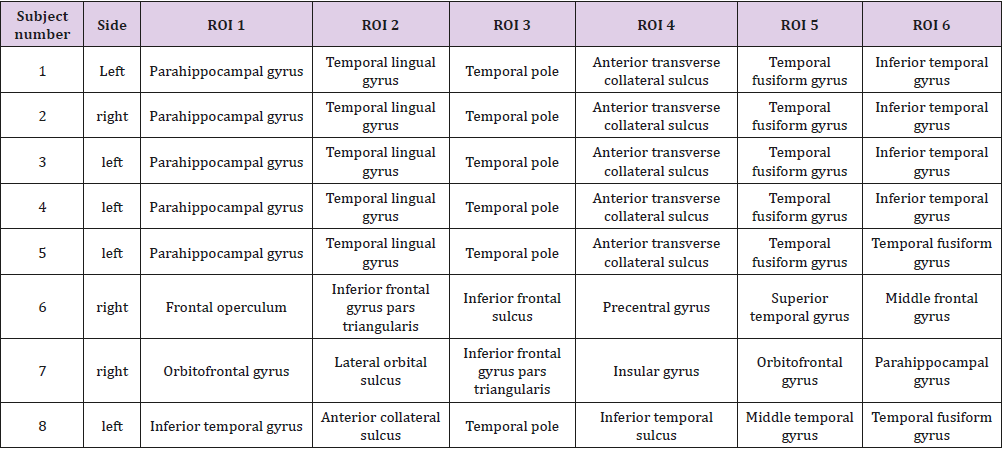
Significant differences of longitudinal changes connectivity (PLV of pre-op MEG state minus PLV of post-op MEG (PLV calculated setting the channel most closely located in the probable epileptogenic lesion as region of interest and the remaining brain areas, and go beyond the predefined threshold) between seizurefree patients and subjects with persistent seizures were observed (p < 0.05; (Figure 2), upper panels). The longitudinal change of whole brain connection numbers (numbers of connection of average PLV values embracing whole brain of each frequency band above the threshold) were not different in the two groups (Figure 2), lower panels).
Figure 2: Region of interest-based connectivity (upper panel) and whole-brain connectivity (lower panel). The left row depicts connectivity analysis of pre-op MEG, while the right row is the results from post-op MEG.
Discussion
Our preliminary result on pre-operative and post-operative MEG with temporal lobe epilepsy patients showed that poor surgical outcome after surgical treatment is related with persistent or increase of connectivity between area surrounding resected lesion and remaining brain regions. Duration of illness was slightly longer in patients with recurred seizures and seizure duration is known to be related with outcome of temporal lobectomy according to older studies [16,17]. But considering that pre-surgical connectivity map showed similar degree of inter-regional connectivity in patients with either longer or shorter disease duration, the effect of seizure duration might only partly explain the difference of seizure outcome. Despite of heterogeneity of pathology reports in our patients, specific surgical pathology is not related with worse surgical outcome even though diffuse or no obvious abnormality is a risk factor for a seizure recurrence [18]. No focal pathologic diagnosis was made in one patient in each group, respectively. Considering that generally poorer prognosis is reported in patients with no histologic diagnosis or pathology other than classical hippocampal sclerosis, a careful caution is needed when interpreting our result on data with heterogenous pathology findings.
Although we did not observe difference of spectral power between the two groups that reach statistical significance, a tendency for decrease of delta power in areas (ROI 6) in seizurefree patients compared to ones with persistent seizures. A previous study on EEG free from visual abnormality showed difference of spectrum-based metrics between TLE and healthy subjects [19]. Because interictal lesional slowing is related with epileptic network in TLE patients [20], our data suggest that still remaining abnormal network is related with recurrence of seizures. A recently published paper presented comparable finding to ours, in which increase of MEG delta activity was noted in patients with unsuccessful epilepsy surgery [14]. Synaptic reorganization of both glutamatergic and GABAergic networks in epileptic brain region even following temporal lobe surgery might explain increase of connectivity in patients with seizure recurrence. The higher interregional correlation might reflect localized physiologic dysfunction even in the absence of spikes [1,21]. These findings are also shown in other neuroimaging studies [22,23].
Our study has few limitations. First, despite of heterogeneity in surgical pathology, clinical and demographic variables, we analyzed very limited number of subjects. Therefore, a lack of statistical power due to small number of patients is of a main issue. Second, it should be noted that cognitive function of the subjects was not included in our current study. Considering that the functional connectivity changes of epilepsy surgery are known to be related with functional reorganization of cognitive network, further studies including pre- and post-surgical cognitive function will be needed. Third, we could not observe changes in very long term (up to 10 years) follow-up of temporal lobectomy, because seizure prognosis after temporal lobectomy is often variable [24](Table 2).
Table 2: Regions of interest for spectral power analysis (peri-lesional area exhibiting greatest source localization of interictal spikes, in order of proximity to the lesion).
Conclusion
The findings suggest that favorable prognosis is associated with decrease of local connectivity in patients with TLE. For more favorable surgical outcome in TLE surgery, resection of not only the epileptogenic structural lesion but also normalization of abnormal connection might be important. Also, further studies are needed to assess a causal relationship between surgical outcome and connectivity changes.
Acknowledgement
This research was supported by grant provided to Won Seok Chang by a faculty research grant of Yonsei University College of Medicine (6-2019-0116). Kyoo Ho Cho was supported by a faculty research grant from Yonsei University College of Medicine 6-2018- 0092.
Conflicts of Interest
The authors declare that the research was conducted in the absence of any personal, commercial, or financial relationships that could potentially be construed as a conflict of interest.
Source of Funding
None.
Author Contributions
Kyoo Ho Cho, analysis and interpretation of data, writing of original draft. Su Jung Hwang, acquisition of data and MEG data analysis. Bong Soo Kim, interpretation of data and critical revision of the manuscript for intellectual content. Kyoung Heo, interpretation of data and critical revision of the manuscript for intellectual content. Jin Woo Chang, study concept and design, critical revision of the manuscript for intellectual content. Won Seok Chang, study concept and design, analysis and interpretation of data, critical revision of the manuscript for intellectual content.
References
- Gallen CC, Tecoma E, Iragui V, Sobel DF, Schwartz BJ, et al. (1997) Magnetic source imaging of abnormal low-frequency magnetic activity in presurgical evaluations of epilepsy. Epilepsia 38(4): 452-460.
- Spencer SS (1996) Long-term outcome after epilepsy surgery. Epilepsia 37(9): 807-813.
- Jeong SW, Lee SK, Hong KS, Kim KK, Chung CK, et al. (2005) Prognostic factors for the surgery for mesial temporal lobe epilepsy: longitudinal analysis. Epilepsia 46(8): 1273-1279.
- Rocha DACARA, Crociati Meguins L, R DESDT, Pongeluppi RI, DA Silva SC J, et al. (2017) Prognostic factors for temporal lobe epilepsy surgery in a tertiary center. Journal of neurosurgical sciences 61(2): 157-163.
- Stufflebeam SM (2011) Clinical magnetoencephalography for neurosurgery. Neurosurgery clinics of North America 22(2): 153-167.
- Cohen D, Cuffin BN (1983) Demonstration of useful differences between magnetoencephalogram and electroencephalogram. Electroencephalography and clinical neurophysiology 56(1): 38-51.
- Murakami H, Wang ZI, Marashly A, Krishnan B, Prayson RA, et al. (2016) Correlating magnetoencephalography to stereo-electroencephalography in patients undergoing epilepsy surgery. Brain : a journal of neurology 139(11): 2935-2947.
- Ito T, Otsubo H, Shiraishi H, Yagyu K, Takahashi Y, et al. (2015) Advantageous information provided by magnetoencephalography for patients with neocortical epilepsy. Brain & development 37(2): 237-242.
- Warren CP, Hu S, Stead M, Brinkmann BH, Bower MR, et al. (2010) Synchrony in normal and focal epileptic brain: the seizure onset zone is functionally disconnected. Journal of neurophysiology 104(6): 3530-3539.
- Palmigiano A, Pastor J, Garcia de Sola R, Ortega GJ (2012) Stability of synchronization clusters and seizurability in temporal lobe epilepsy. PloS one 7(7): 41799.
- van Dellen E, Douw L, Hillebrand A, Ris Hilgersom IH, Schoonheim MM, et al. (2012) MEG network differences between low- and high-grade glioma related to epilepsy and cognition. PloS one 7(11): 50122.
- Horstmann MT, Bialonski S, Noennig N, Mai H, Prusseit J, et al. (2010) State dependent properties of epileptic brain networks: comparative graph-theoretical analyses of simultaneously recorded EEG and MEG. Clinical neurophysiology : official journal of the International Federation of Clinical Neurophysiology 121 (2): 172-185.
- van Dellen E, Douw L, Hillebrand A, De Witt Hamer PC, Baayen JC, et al. (2014) Epilepsy surgery outcome and functional network alterations in longitudinal MEG: a minimum spanning tree analysis. NeuroImage 86: 354-363.
- Schonherr M, Stefan H, Hamer HM, Rossler K, Buchfelder M, et al. (2017) The delta between postoperative seizure freedom and persistence: Automatically detected focal slow waves after epilepsy surgery. NeuroImage Clinical 13: 256-263.
- Lachaux JP, Rodriguez E, Martinerie J, Varela FJ (1999) Measuring phase synchrony in brain signals. Human brain mapping 8(4): 194-208.
- Ficker DM, So EL, Mosewich RK, Radhakrishnan K, Cascino GD, et al. (1999) Improvement and deterioration of seizure control during the postsurgical course of epilepsy surgery patients. Epilepsia 40(1): 62-67.
- Eliashiv SD, Dewar S, Wainwright I, Engel J, Fried I, et al. (1997) Long-term follow-up after temporal lobe resection for lesions associated with chronic seizures. Neurology 48(3): 621-626.
- McIntosh AM, Kalnins RM, Mitchell LA, Fabinyi GC, Briellmann RS, et al. (2004) Temporal lobectomy: long-term seizure outcome, late recurrence and risks for seizure recurrence. Brain : a journal of neurology 127 (9): 2018-2030.
- Kalamangalam GP, Cara L, Tandon N, Slater JD (2014) An interictal EEG spectral metric for temporal lobe epilepsy lateralization. Epilepsy research 108(10): 1748-1757.
- Tao JX, Chen XJ, Baldwin M, Yung I, Rose S, et al. (2011) Interictal regional delta slowing is an EEG marker of epileptic network in temporal lobe epilepsy. Epilepsia 52(3): 467-476.
- Baayen JC, de Jongh A, Stam CJ, de Munck JC, Jonkman JJ, et al. (2003) Localization of slow wave activity in patients with tumor-associated epilepsy. Brain topography 16(2): 85-93.
- Keller SS, Richardson MP, Schoene Bake JC, O Muircheartaigh J, Elkommos S, et al. (2015) Thalamotemporal alteration and postoperative seizures in temporal lobe epilepsy. Annals of neurology 77 (5): 760-774.
- Ji GJ, Zhang Z, Xu Q, Wei W, Wang J, et al. (2015) Connectome Reorganization Associated With Surgical Outcome in Temporal Lobe Epilepsy. Medicine 94(40): 1737.
- Sadek AR, Gray WP (2011) Chopping and changing: long-term results of epilepsy surgery. Lancet (London, England) 378(9800): 1360-1362.

 Research Article
Research Article