Abstract
To investigate the protective effect of Edaravone (EDa) against apoptosis induced by Gentamicin (GM) in MDCK cells, MDCK cells were treated with 4mmol/L GM for 24h following pre-incubation with or without EDa (40μmol/L) for 30 min. The apoptotic rate was detected by Annexin V-FITC/PI double staining; and changes in nuclear morphology were observed by Hoechst 33258 staining. T-SOD, GSH-Px, CAT activity and MDA content were detected by colorimetric assays. ROS content was detected by fluorescent staining; the expression of Bax and Bcl-2, the activation of caspase-9, caspase-3 and PARP protein, and the release of Cyt C and AIF were detected by western blotting. The results showed that EDa significantly inhibited GM-induced apoptosis in MDCK cells. EDa induced changes in nuclear morphology and ROS content and caused decreased T-SOD and GSHPx activity, increased CAT activity, decreased MDA content, increased Bcl-2/Bax ratio, inhibited activation of caspase-9, caspase-3 and PARP protein, and release of Cyt C and AIF (P<0.05 or P<0.01). These findings suggest that EDa can relieve oxidative damage to GM-induced apoptosis through the mitochondrial apoptotic pathway.
Keywords: Gentamicin; MDCK Cells; Mitochondrial Apoptosis; Edaravone
Abbreviations: GEda: Edaravone, GM: Gentamicin, ROS: Reactive Oxygen Species, CAT: Catalase, SOD: Superoxide Dismutase, MDA: Malondialdehyde, DMEM: Dulbecco’s Modified Eagle’s Medium, FBS: Fetal Bovine Serum, PBS: Phosphate Buffered Saline, BCA: Bicinchoninic Acid
Introduction
The kidney is an important excretory organ of the body, and tubule cells are the basic structural and functional unit that constitutes the kidney. Gentamicin (GM), an aminoglycoside antibiotic, is widely used in clinical practice. The doses of gentamicin for adults are given 1-1.7mg/kg every 8 hours; or 0.75- 1.25mg/kg per 6 hours1. Children are given 3-5mg/kg a day and administered 2-3 times [1]. When used improperly, it accumulates in the bodies of animals, and its main targets are the kidney and ear tissues [2]. Studies have shown that GM can cause damage and apoptosis of rat tubule cells through oxidative stress [3-5]. Free radicals found in the body mainly include Reactive Oxygen Species (ROS), alipid free radicals and lipid peroxidation free radicals. Under normal conditions, ROS in the body are in a state of dynamic balance between production and consumption. When the body is dreceives external stimuli, the accumulation of ROS causes damage to proteins, biofilms and DNA [6].
Edaravone (EDa), a synthetic antioxidant, is fat- soluble and can pass through the blood-brain barrier [7]. EDa protects cells by eliminating ROS from multiple types of cells and by reducing oxidative stress [8-10]. EDa has mainly been used to treat acute cerebral infarction and hypoxic ischemic encephalopathy [10]. However, whether it inhibits the apoptosis of MDCK cells through antioxidation has not been widely studued. In this study, the effects of GM and EDa on the apoptotic rate of cells and the activity of Superoxide Dismutase (SOD), Glutathione Peroxidase (GSH-Px), Catalase (CAT), and Malondialdehyde (MDA) were examined. The expression of mitochondrial apoptosis pathway-associated proteins was detected by western blot to explore the protective effect of EDa in GM- induced MDCK cell apoptosis.
Materials and Methods
All chemicals were of the highest-grade purity available. Eda was purchased from Tocris Bioscience (Ellisville, MO, USA). GM, penicillin, Hoechst 33258 and streptomycin were purchased from Sigma Aldrich (St. Louis, MO, USA). Dulbecco’s Modified Eagle’s Medium (DMEM), Fetal Bovine Serum (FBS) and trypsin were obtained from Gibco (Grand Island, NY, USA). Accutase cell detachment solution and an Annexin V–fluorescein isothiocyanate/propidium iodide (FITC/PI) apoptosis detection kit was purchased from Becton Dickinson (San Diego, CA, USA). A reactive oxygen detection kit was purchased from Beyotime Biotechnology (Shanghai, China). T-SOD, MDA, CAT, and GSH-Px kits were from Nanjing Jiancheng Bioengineering Institute (Nanjing, China). All other chemicals were from Sigma-Aldrich. The following primary antibodies were used: anti-Bax(CST, #14796), anti–cytochrome (cyt c) (CST, #14940), anti–cleaved caspase-9 (CST, #9506), anti–cleaved caspase-3 (CST, #9664), and anti–β-actin (CST, #4970S) were from Cell Signaling Technology (Boston, USA); anti-AIF (Abcam, ab1998), anti-cleaved PARP(Abcam, ab32064) and anti-Bcl-2(Abcam, ab32124) were from Abcam (Cambridge, USA). All secondary antibodies were from Cell Signaling Technology (Boston, USA).
Drugs
The dosage of gentamicin in this study is a model for nephrotoxicity and previously used by Servais H [11]. and Li X13. The dosage of edaravone in this study is previously used by Liu G14.
Cell Culture
The MDCK cell line was purchased from the Type Culture Collection of the Chinese Academy of Sciences (Shanghai, China). MDCK cells were cultured in DMEM supplemented with 10% FBS at 37 °C in 5% CO2.
Detection of Apoptosis
MDCK cells (5×105 cells/well) were cultured in six-well plates. After 30min of preincubation with EDa (40μmol/L), cells were cotreated with or without GM (4mmol/L) for 24h. Then, the cells were resuspended in 200μl 1×binding buffer containing 5μl Annexin V-FITC and 5μl PI for 15 min at room temperature in the dark. The cells were analyzed using a FACSAria flow cytometer (Becton Dickinson, San Jose, CA, USA). The percentage of apoptosis was considered the sum of from Annexin V+/PI− and annexin V+/ PI+ fluorescence.
Hoechst 33258 Staining
Apoptotic morphological changes in the nuclear chromatin of MDCK cells were examined using Hoechst 33258 staining, as previously described. MDCK cells (5×104 cells per well) were cultured in 12-well plates. After 30 min of preincubation with EDa (40μmol/L), cells were cotreated with or without GM (4mmol/L) for 24 h. Then, cells were washed with ice-cold Phosphate Buffered Saline (PBS), fixed with paraformaldehyde (4% w/v) for 20 min at room temperature, and incubated with Hoechst 33258 staining solution (5mg/L in PBS) for 15 min at room temperature in the dark. After washing in PBS three times, the cells were viewed under a Leica inverted fluorescence microscope (Wetzlar, GER) at an excitation wavelength of 352nm. To assess the extent of GM-induced apoptosis, 200 cells per experiment were randomly selected and the apoptotic cells were counted; each experiment was performed in triplicate.
ROS Measurement
MDCK cells (5×104 cells per well) were cultured in 12-well plates. After 30 min of preincubation with EDa (40 μmol/L), cells were cotreated with or without GM (4mmol/L) for 24 h. Followiing treatment, cells were incubated with DCFH-DA staining for 20 min at 37℃ in the dark and washed three times with PBS. The fluorescence intensity was measured by using a flow cytometer at an excitation wavelength of 488nm.
Oxidative Stress Assessment
The cells were extracted to determine the concentrations of the antioxidant enzymes present. The procedures were conducted in accordance with the instructions provided in the kit from Nanjing Jiancheng Bioengineering Institute. The detection kit can be used to measure the levels of SOD (SOD Assay Kit with WST-8), GSHPx (DTNB colorimetric method), CAT (ammonium molybdate colorimetry) and MDA (thiobarbituric acid colorimetric method).
Western Blot Analysis
Protein concentrations were assayed using a Bicinchoninic Acid (BCA) protein assay kit (Beyotime, Shanghai, China). All proteins were separated by 8-15% SDS-polyacrylamide gel electrophoresis and transferred to 0.22 μm PVDF membranes. Then, the membranes were blocked in 5% skim milk in TBST for 1.5h at room temperature before incubation with primary antibodies for Bax, Bcl-2, cleaved caspase-9, cleaved caspase-3, cleaved PARP, Cyt C, AIF, and β-actin (1:1000) overnight at 4°C and then with the appropriate secondary antibodies (1:5000) for 2 h at room temperature. Protein levels were determined by standard scanning densitometry with normalization to β-actin. All assays were performed in triplicate.
Statistical Analysis
All data are expressed as the mean ± standard deviation, and significance was determined by one-way ANOVA, followed by LSD’s test using SPSS 20.0 (SPSS, Chicago, USA). The results were considered significant at P < 0.05 and highly significant at P < 0.01.
Results
Apoptosis of MDCK cells
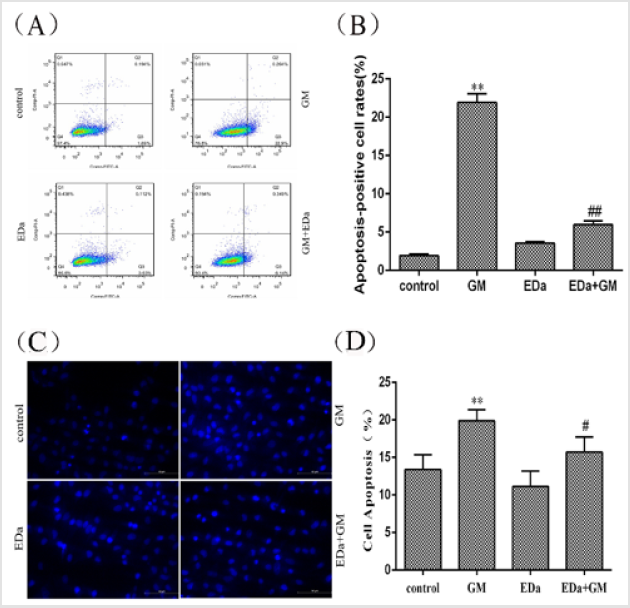
In this study, the effects of GM on apoptosis were distinguished by Annexin V-FITC/PI double staining and Hoechst 33258 staining (Figure 1). After 24 h of incubation with GM (4mmol/L), GM ap peared to markedly alter the nuclear morphology and apoptosis rate. However, EDa prevented GM-induced increases in the apoptosis rate and the appearance of nuclear morphological changes typical of apoptosis.
Figure 1: Effects of GM and EDa on the apoptosis rate in MDCK cells.
(A) The results of flow cytometry.
(B) Statistical results.
(C) Morphological changes.
(D) Apoptosis rates. Compared with the control group, **P<0.01; compared with the respective GM treatment group,
#P<0.05, ##P<0.01. Scale bar = 50μm. GM, gentamicin; EDa, edaravone.
The level of Oxidative Stress in MDCK Cells
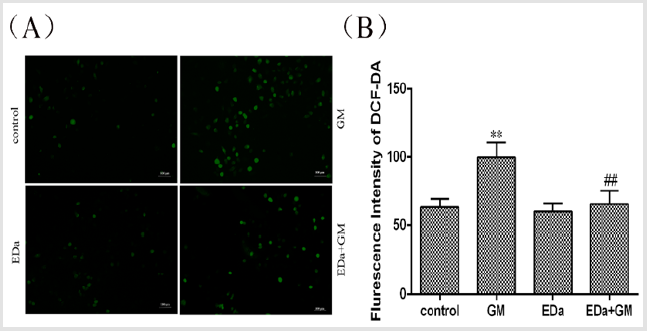
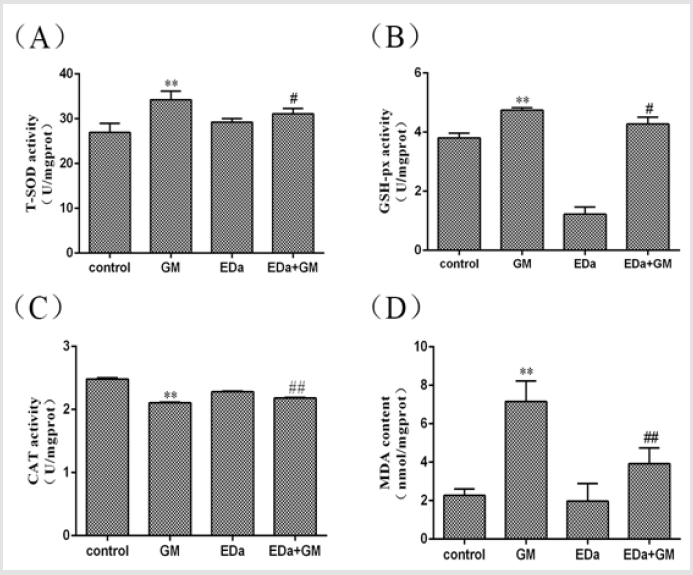
MDCK cells were treated with 4mmol/LGM for 24h following preincubation with or without EDa (40μmol/L) for 30min. The level of oxidative stress is shown in (Figures 2 & 3). The levels of ROS and MDA and the activities of T-SOD and GSH-Px were significantly higher, but the activity of CAT was markedly lower in the GM group, whereas the levels of ROS and MDA tended to decrease and the antioxidant activity was significantly lower in the GM+EDa group than in the GM group. The EDa group showed no significant differences in these indicators compared with the control group.
Figure 2: Effect of GM and EDa on the level of oxidative stress in MDCK cells.
(A) The results of fluorescence detection.
(B) Statistical analysis of the fluorescence results. Compared with the control group, **P<0.01; compared with the GM
treatment group, ##P<0.01. Scale bar = 50μm. GM, gentamicin; EDa, edaravone.
Figure 3: Antioxidative enzyme activity and MDA levels in MDCK cells.
(A) The activity of T-SOD.
(B) The activity of GSH-Px.
(C) The activity of CAT.
(D) The level of MDA. Compared with the control group, **P<0.01; compared with the GM treatment group, #P<0.05,
##P<0.01. GM, gentamicin; EDa, edaravone.
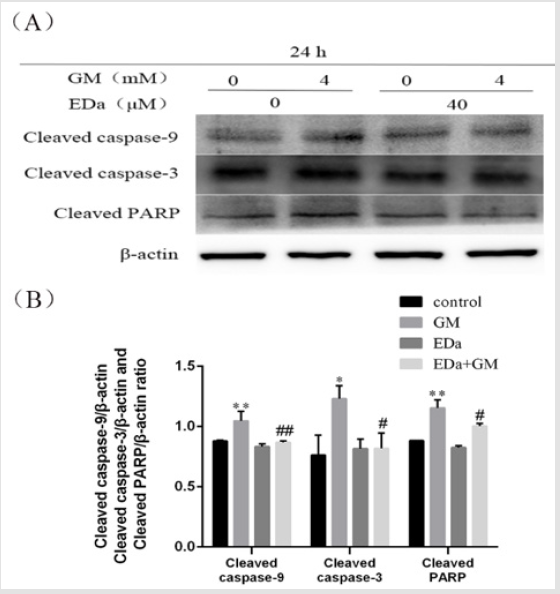
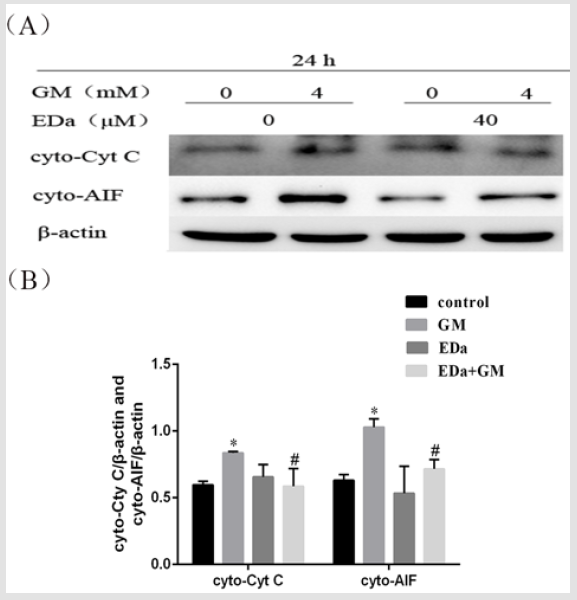
Expression of Apoptosis-Related Proteins in MDCK Cells
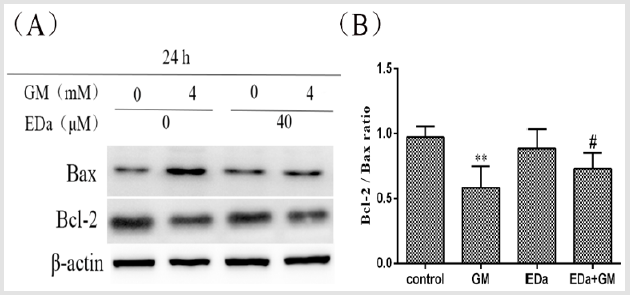
MDCK cells were treated with 4mmol/L GM for 24h following preincubation with or without EDa (40μmol/L) for 30min. Then, the protein activity of Bax, Bcl-2, cleaved caspase-9, cleaved caspase-3, cleaved PARP, cty C and AIF in the mitochondrial apoptosis pathway was detected by western blot (Figures 4-6). In the GM group, the levels of Bax, cleaved caspase-9, cleaved caspase-3, cleaved PARP, cty C and AIF were significantly increased, but the Bcl-2/Bax ratio was decreased. Moreover, in the GM+EDa group, the levels of Bax, cleaved caspase-9, cleaved caspase-3, cleaved PARP, cty C and AIF were significantly decreased and the Bcl-2/Bax ratio was increased.
Figure 4: The expression levels of Bax and Bcl-2.
(A) The expression levels of Bax and Bcl-2 in MDCK cells were detected by western blot analysis.
(B) Statistical analysis of the western blot results of three independent experiments (mean ± SD, n = 3). Compared with the
control group, **P<0.01; compared with the GM treatment group, #P<0.05. GM, gentamicin; EDa, edaravone.
Figure 5: The levels of cleaved caspase-9, cleaved caspase-3 and cleaved PARP in MDCK cells.
(A) The levels of cleaved caspase-9, cleaved caspase-3 and PARP were detected by western blot analysis.
(B) Statistical analysis of tshe results of three independent experiments (mean ± SD, n = 3). Compare with control group,
*P<0.05, **P<0.01; compare with respective GM treatment group, # P<0.05, ##P<0.01. GM, gentamicin; EDa, edaravone.
Figure 6: The release of Cyt C and AIF from MDCK cells.
(A) The release of Cyt C and AIF was detected by western blot analysis.
(B) Statistical analysis of the results of three independent experiments (mean ± SD, n = 3). Compared with the control group,
*P<0.05; compared with the GM treatment group, #P<0.05. GM, gentamicin; EDa, edaravone.
Discussion
GM is a commonly used aminoglycoside antibiotic used in animal clinical practice, but due to its toxic effects, the incidence of kidney damage is approximately 36% [15]. GM was injected intraperitoneally at a dose of 100 mg/kg for seven successive days, which is well known to cause significant nephrotoxicity in rats [16]. Many studies have shown that GM can induce an increase in ROS [16-18].and oxidative stress in various cells [19-21]. Excessive ROS can disrupt mitochondrial membrane integrity, cause a decrease in mitochondrial membrane potential, increase membrane permeability, and promote mitochondrial release of apoptosisrelated proteins. In cochlear hair cells, GM induced apoptosis through ROS accumulation [22].
ROS are mitochondrial respiratory products that have a high degree of oxidative activity [23]. Under normal physiological conditions, ROS can regulate intracellular signal transduction, but excessively high concentrations of ROS will induce oxidative stress in cells, causing apoptosis or necrosis of cells. In a variety of cells, there is a close relationship between oxidative stress and apoptosis. Oxidative stress not only directly induces apoptosis but also mediates apoptosis by regulating genes and related signal transduction [24-26]. Experiments have confirmed that oxidative stress is involved in the death receptor pathway, the mitochondrial pathway and endoplasmic reticulum stress-mediated apoptosis [25-28]. The results of this study showed that GM increased the ROS level and activated the cellular antioxidant enzyme system. It also increased the Bcl-2/Bax ratio, the expression of cleaved caspase-9, cleaved caspase-3, cleaved PARP protein, release of Cyt C and AIF. These fingings suggest that the accumulation of ROS may activate the mitochondrial pathway in GM-induced apoptosis of MDCK cells.
EDa, as an antioxidant used in clinical practice, can effectively reduce the level of ROS in the body [29,30]. A model of HT22 cell injury can be induced by hydrogen peroxide; EDa can antagonize oxidative stress, reduce ROS levels, downregulate Bax expression, and upregulate Bcl expression [31]. This study revealed that pretreatment with EDa significantly inhibited ROS levels and altered in antioxidant enzyme activity. Furthermore, Bcl-2 expression was increased, and Bax and mitochondrial apoptosis pathway proteins were decreased, which confirmed that EDa can inhibit activation of the mitochondrial apoptosis pathway by scavenging ROS.
Conclusion
The present study suggested that the accumulation of ROS plays an important role in GM-induced apoptosis of MDCK cells and that EDa can confer a marked protective effect against GM-induced oxidative stress and nephrotoxicity. Our findings suggest that preincubation with EDa may be effective in clinical applications given its with antioxidant properties. However, further investigations are essential to elucidate the exact mechanism of protection and the potential usefulness of EDa as a protective agent against drug toxicity in clinical trials.
References
- Xiao YH (2004) Clinical antibiotics. Chongqing publisher, China.
- Antoine DJ, Srivastava A, Pirmohamed M (2010) Statins inhibit aminoglycoside accumulation and cytotoxicity to renal proximal tubule cells. Biochemical Pharmacology 79(4): 647-654.
- Adil M, Kandhare AD, Dalvi G, Raygude KS, Bodhankar SL, et al. (2016) Ameliorative effect of berberine against gentamicin-induced nephrotoxicity in rats via attenuation of oxidative stress, inflammation, apoptosis and mitochondrial dysfunction. Renal Failure 38(6): 996-1006.
- Kandemir FM, Ozkaraca M, Yildirim BA, Aktas E, Benzer F, et al. (2015) Rutin attenuates gentamicin-induced renal damage by reducing oxidative stress, inflammation, apoptosis, and autophagy in rats. Renal Failure 37(3): 518-525.
- Liu C, Kang Y, Qin F, Gu J, Han C, et al. (2017) Rhizoma smilacis glabrae protects rats with gentamicin-induced kidney injury from oxidative stress-induced apoptosis by inhibiting caspase-3 activation. Journal of Ethnopharmacology 198: 122-130.
- Simon HU, Haj Yehia A, Levi Schaffer F (2000) Role of reactive oxygen species (ROS) in apoptosis induction. Apoptosis 5(5): 415-418.
- Watanabe T, Tahara M, Todo S (2008) The novel antioxidant edaravone: from bench to bedside. Cardiovascular Therapeutics 26(2): 101.
- Matsumoto S, Hanai T, Shimizu N (2010) Effect of edaravone on ischemia/reperfusion injury in rat urinary bladder-changes in smooth muscle cell phenotype and contractile function. Aktuelle Urologie 41 Suppl 1(1): S46.
- Li Y, Liu H, Zeng W, Wei J (2017) Edaravone protects against hyperosmolarity-induced oxidative stress and apoptosis in primary human corneal epithelial cells. Plos One 12(3): e0174437.
- Li Q, Bi MJ, Bi WK, Yuan Jiang (2016) Edaravone attenuates brain damage in rats after acute CO poisoning through inhibiting apoptosis and oxidative stress. Environmental Toxicology 31(3): 372-379.
- Hélène Servais, Smissen PVD, Thirion G (2005) Gentamicin-induced apoptosis in LLC-PK1 cells: Involvement of lysosomes and mitochondria. Toxicology & Applied Pharmacology 206(3): 321-333.
- Hélène Servais, Jossin Y, Françoise Van Bambeke, Tulkens PM, Mingeot Leclercq MP, et al. (2006) Gentamicin Causes Apoptosis at Low Concentrations in Renal LLC-PK1 Cells Subjected to Electroporation. Antimicrobial Agents & Chemotherapy 50(4): 1213-1221.
- Li X, Zheng FL, Liu YX (1998) Apoptosis in gentamicin treated LLC-PK1 cells and its relationship with intracellular calcium levels. Chinese Journal of Internal Medicine 37(10): 667-670.
- Liu G, Guo R, Wenming XU (2014) Edaravone protects H9 c2 cells against doxorubicin-induced cardiotoxicity. Chinese Pharmacological Bulletin 30(4): 490-495.
- Kleinknecht D, Landais P, Goldfarb B (1987) Drug-Associated Acute Renal Failure. A Prospective Collaborative Study of 81 Biopsied Patients. Acute Renal Failure. Springer US 212: 125-128.
- Wiland P, Szechcinski J (2003) Proximal tubule damage in patients treated with gentamicin or amikacin. Pol J Pharmacol 55(4): 631-637.
- Karahan I, Ateşşahin A, Yilmaz S, Ceribaşi AO, Sakin F (2005) Protective effect of lycopene on gentamicin-induced oxidative stress and nephrotoxicity in rats. Toxicology 215(3): 198-204.
- Bustos PS, Deza Ponzio R, Páez PL, Virgolini MB, Ortega MG, et al. (2018) Flavonoids as protective agents against oxidative stress induced by gentamicin in systemic circulation. Potent protective activity and microbial synergism of luteolin. Food & Chemical Toxicology an International Journal Published for the British Industrial Biological Research Association 118: 294-302.
- Zhang M, Shen J, Association HM (2015) Protection of Edaravone on Oxidative Stress and Apoptosis of Renal Tubular Epithelial Cells in Rats. Herald of Medicine (7): 875-878.
- Denu RA, Hematti P (2016) Effects of Oxidative Stress on Mesenchymal Stem Cell Biology. Oxidative Medicine & Cellular Longevity 1: 1-9.
- Zhou J, GE L, JIA C (2016) ROS-mediated Different Homeostasis of Murine Corneal Epithelial Progenitor Cell Line under Oxidative Stress. Scientific Reports 6: 36481.
- Wooltorton E (2002) Ototoxic effects from gentamicin ear drops. CMAJ: Canadian Medical Association journal journal de l'Association medicale canadienne 167(1): 56.
- Ott M, Gogvadze V, Orrenius S (2007) Mitochondria, oxidative stress and cell death. Apoptosis 12(5): 913-922.
- Yang C, Yang G, Xu S, Jiyao Jiang, Joseph H, et al. (2016) Glutamate carboxypeptidase II gene knockout attenuates oxidative stress and cortical apoptosis after traumatic brain injury. Bmc Neuroscience 17(1): 15.
- Ozben T (2007) Oxidative stress and apoptosis: impact on cancer therapy. Journal of Pharmaceutical Sciences 96(9): 2181-2196.
- Matés J, Segura J, Alonso F (2000) Oxidative Stress and Apoptosis.
- Qi F, Li A, Inagaki Y (2012) Induction of apoptosis by cinobufacini preparation through mitochondria- and Fas-mediated caspase-dependent pathways in human hepatocellular carcinoma cells. Food & Chemical Toxicology an International Journal Published for the British Industrial Biological Research Association 50(2): 295-302.
- Morio Y, Tsuji M, Inagaki M, Oguchi K, Furuya K, et al. (2013) Ethanol-induced apoptosis in human liver adenocarcinoma cells (SK-Hep1): Fas- and mitochondria-mediated pathways and interaction with MAPK signaling system. Toxicology in Vitro An International Journal Published in Association with Bibra 27(6): 1820-1829.
- MI IR, Murakami Y, Thanos A, Vavvas DG, Miller JW (2011) Edaravone, an ROS Scavenger, Ameliorates Photoreceptor Cell Death after Experimental Retinal Detachment. Investigative Ophthalmology & Visual Science 52(6): 3825.
- Watanabe T, Tahara M, Todo S (2008) The novel antioxidant edaravone: from bench to bedside. Cardiovascular Therapeutics 26(2): 101-114.
- Zhao ZY, Luan P, Huang SX, Liu J (2013) Edaravone protects HT22 neurons from H2O2-induced apoptosis by inhibiting the MAPK signaling pathway. Cns Neuroscience & Therapeutics 19(3): 163-169.

 Research Article
Research Article