Abstract
Heterocyclic compounds especially those with Oxygen and Nitrogen atoms have shown many applications in chemotherapy as anti-cancer drug, anti-depression, anti-viral, anti-microbial as well as many other medical applications. In our investigation we use ultrasonic technique for preparing heterocyclic compounds mainly compounds E4, E5-7, E8-14, E15-18 and E19-22. Compounds E4 was prepared by condensation of meta toluidine with diabromo acrylyl chloride and cyclization with thiouria while compounds E5-7 were derived from either furfural as dibromo furfural on condensation with dimedon , compounds E8-14 were synthesized by condensation of dibromo furfural with acetone , urea , thiouria and sulfuric acid while compounds E15-18 and E19-22 were prepared from condensation of, α, β- Naphthol and urea using zarconyl chloride. The synthesized compound were identified by IR, NMR and were discussed.
Keywords: Heterocyclic compounds; Furfural; Ultrasonic waves
Introduction
Furfural was first time produced industrially from rice huks in1840 after drying mixing with sodium chloride and addition of 10% H2SO4 and distilled water [1], Other researchers have synthesized it from rice straw in 2007 [2]. Punsuvon and his coworkers have synthesized furfural from sugar cane stalks and sulfuric acid [3] with an overall yield of 71%. In 2010 researchers have succeeded to synthesize furfural from xylose sugar [4]. In 2012 other researchers have prepared furfural from epic rap of wild mango [5]. In 2016 researchers have prepared furfural from bagasse [6] According to the above works it was known that furfural is cheap precursor and was used for the synthesis of variety of heterocyclic compounds. Furfural itself and its derivatives MCA, MBA for example 4,5-Dibromofurfuraldehydle, 2-(2-furyl) [1,3] dioxane,5- nito(1,3-imidazolyl-2,5-dion)-3-yl furfuraldine was used as drag in treatment of urinary tract [7,8]. Among the reactions of furfural are the synthesis of tetrazine derivatives [9], furyl methylene diacetate [10] and 4-methyl furfural [11]. Among the known reactions of furfural which leads to the formation of heterocyclic compounds are the synthesis of 1,3-imidizolyl-2,5- furfuryl amine-2,s5- dione which is used for treatment of urinary tract infections [12]. Furoin compound on oxidation forms furil which is known as insect side [13]. flavon compound contains furfural ring, furfuraldehyde exhibited ICso values of 75.9,51.0 and 59.3 M for HT29, MCF7 and A498 respectively as anti-cancer cell lines [14]. It was also known that bromo derivative of furfural (MBA) reacts with boronyl indole to form indolyl derivatives of furfural, which is known for treatment of prostate, stomach, pancreatic, kolon cancer types [15]. In our investigation, We started from furfural as precursor for the synthesis of some heterocyclic compounds in continuing of our previous study [16-18] for the preparing of new derivatives of this type of furyl compounds in drug discovery program.
Experimental
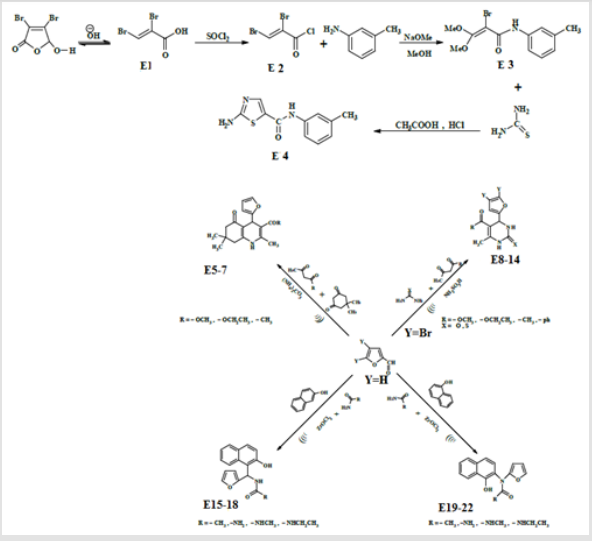
All melting points were uncorrected and measured using Electro thermal melting point apparatus, All chemical were supplied by Aldrich and fluka and BDH companies. Bruker Avance 111 400 MHz was used for 1HNMR measurements. Infrared spectrophotometer model FT (600) CO. LTD (UK) and FT (8400 s) shimadzo were used for IR measurements. power sonic 405 micro process-controlled bench –top ultra-sonic cleaner was used for Ultrasound chemical condensation. Dibromo acrylic acid and its chloride derivative E1, E2 were prepared according to the published procedure [19]. Dibromo furfural and mucobromic acid were prepared following the published procedure [20,21].
Synthesis of 2-Bromo –N- (3-ethyl phenyl)-3, 3-dimethyl propion amide (E3)
A mixture of 1.87 g. of KHCO3 in 10 ml of water was mixed with (1.18 g., 0.01 mol.) of meta toludine in 5 ml of THF. And stirred at r.t for 1 hr. at 60-65ºC, after that 4.22 g. of 75% solution of compound E2 in THF was added gradually within 2hr. while the mixture was then stirring. after complete addition 10 ml of THF was then added and the stirring was continued for further 2 hr. at the same temperature after that the solvent was evaporated and to the residue was added 1.98 g of 30% methanolic sodium methoxide within a period of 1 hr. stirring was continued for further 3hr. evaporation of methanol gave an oil product 57% which was used in next step.
Synthesis of 2-Amino thiazole -5- (3-methyl phenyl) Carboxy Amide(E4)
Compound E3 (1.5 g ,.0048 mol.), 3.5 ml of acetic acid and 1.09 g. of HCL were mixed together and stirred at 60-65ºC , then 0.92 g. of thiouria was added , the stirring was continued for 11 hr. at the same temperature. The reaction was subject for distillation to distill the excess acetic acid methanol 8.47 ml was then added together with 1.35 g. of 30% methanolic sodium methoxide until pH of the mixture becomes 8-9. The reaction mixture was filtered off, to the filtrate was added 2g. of activated charcoal and stirred at 60 ºC for 1 hr., filtered evaporation of the solvent, 20ml of cold water was then added to the residue. The final mixture was cooled to 0ºC. the yellow oil was extracted with ether, the extract was evaporated to give oil product 55%.
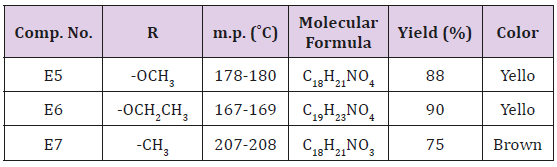
Synthesis of Some Furyl Substituents of polyhydroquinoline (E5-7)
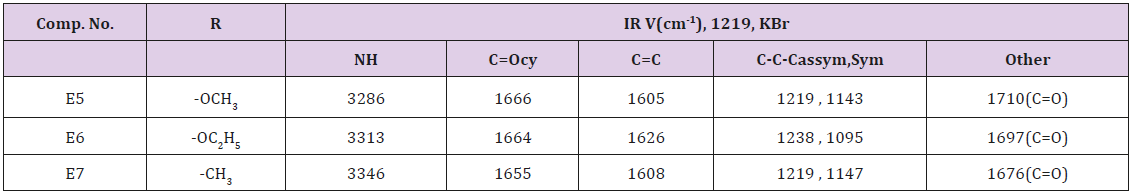
A mixture of (0.96 g., 0.01 mol.) of furfural, (1.4, 0.01 mol.) of dimedon, (0.015 mol.) of ammonium carbonate and (0.013 mol.) of either methyl acetoacetate or ethyl acetoacetate or acetyl acetone in 30 ml of water. The final mixture was sonicated at 60ºC for 1 hr. After complete reaction (TCL) the reaction was cooled and filtered, washed with water and with 25ml of 50% Ethanol. The p.pt was recrystallized from ethanol. physical data were presented in Table 1.
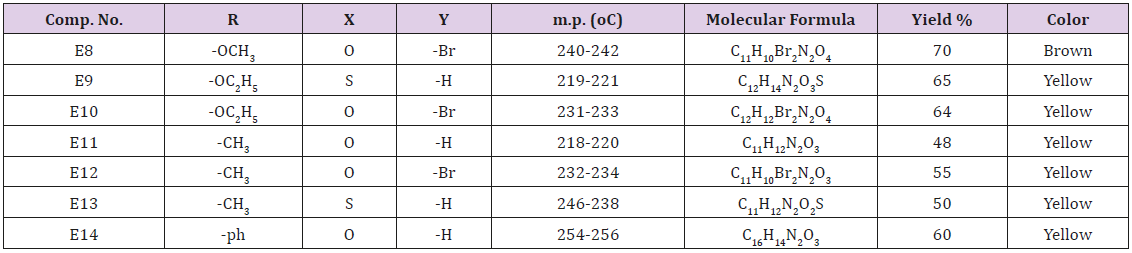
Synthesis of Some Furyl Substituents of 3,4 dihydroprymidine -2-one(E8-14)
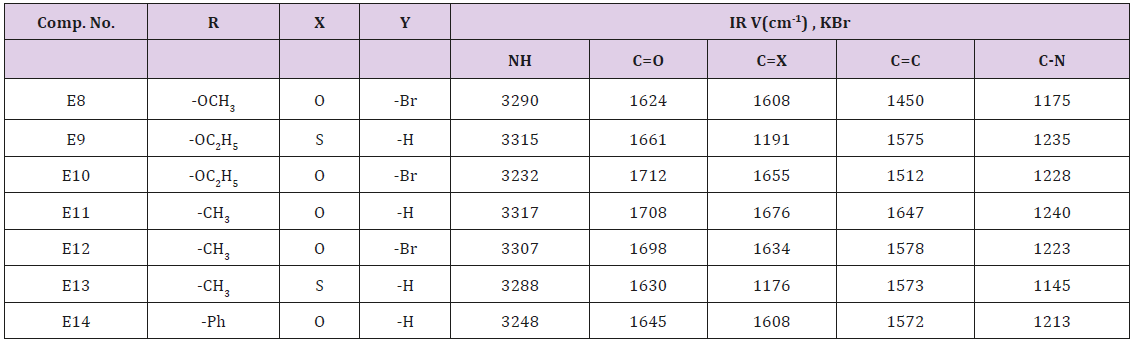
Urea or thiouria and dibromo furfural (0.015 mol.) were mixed together. To the mixture was added methyl or ethyl acetoacetate or acetyl acetone or benzoyl acetone, 50ml of ethanol and 0.08 mol. Of sulfuric acid. The final mixture was irradiated with ultrasound at 25-30ºC for 45 min. the reaction was monitored by TLC. After completion of the reaction the mixture was filtered off. The residue was washed with water then with ethanol, dried and recrystallized from 95% ethanol physical and data are illusterated in Table 2.
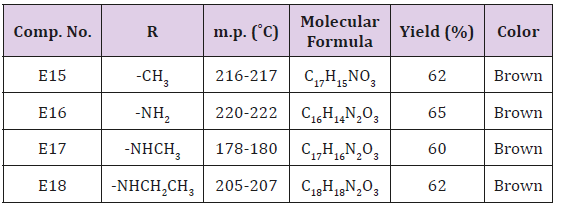
Synthesis of some furan substituents of amino alkyl naphthol (E15-18)
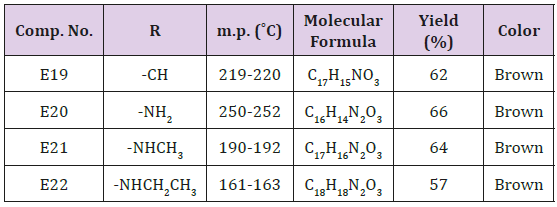
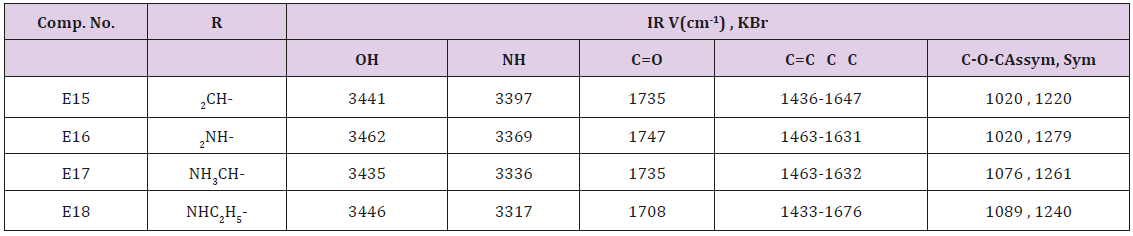
Furfural (0.69 g., 0.01 mol.), 0.01 β-naphthol, 0.01mol. of acetamide or urea or methyl urea and (0.01 mol.) of ZrOCl2. 8H2O in 50 ml of 1,2 – dichloro ethane. The final solution was sonicated at r.t for 40 min. after complete reaction (TLC monitoring), The mixture was filtered off, washed with ether then with water, dried and recrystallized from methanol. physical and spectral data are illustrated in Table 3. The same above procedure was used for synthesizing of compound E20-23 at 60ºC and sonication for 30 min. The crude product was recrystallized from methanol and the physical properties are shown in Table 4.
Result and Discussion
Synthesis of 2-aminothiazole -5-(3-methyl phenyl) carboxy amide (E4)
The first step of this route was the preparation of 2,3-dibromo acrylic acid from the reaction of Mucobromic acid with sodium hydroxide as shown in Scheme 1, the synthesized compound E1 was characterized by the following IR cm-1 3344 for OH , stretching band at 1765 for C=O while C=C appeared at 1624 ,C-O at 1394 ,CBr at 850 .This compound was allowed to react with SOCl2 forming compound E2 which is characterized by the following IR bands cm-1 :C=O at 1786 ,C=C at 1659 ,C-Br at 850 and disappearance of the OH band . Compound E2 was allowed to react with meto toludine forming 2,3-dibromo-N-meta tolyl acryl amide which intern reacts with sodium methoxide result into the formation of E3. This compound was characterized by the following IR cm-1 3378 for NH, 1677 for C=O, Aromatic C=C absorbed between 1457-1605 while C-O appeared at 1312, C-Br at 832. The final steps of this route include the reaction of E3 with thiouria forming E4 which is characterized by the following IR stretching bands cm-1 : 3355 for NH , 1668 for C=O , 1590 for C=N , C=C Aromatic appeared within 1445-1612 , C-S sym. and asym. At 766, 1093 respectively. The amide group test showed positive result.
Synthesis of Some Furyl Substituent of 3,4 –dihydro pyrimidine 2-one, 2-thio (E8-14)
This series of compounds were prepared by ultrasonic irradiation of 4,5-dibromo furfural with uria or thiouria and one of compounds (Methyl, Ethyl acetoacetate, acetyl acetone and benzoyl acetone) see Scheme 1. The synthesized compounds were characterized by the following absorption bands cm-1: 3248-5317 for NH stretching, 1624-1712 for C=O, C=C Aromatic appeared at 1450-1647 while C-N absorbed at 1145-12l40 see Table 6. Compound 9 as representative of the series was characterized by the following resonating signals in ppm. singlet signal at 10.38ppm., 9.62 ppm. for NH protons, doublet signal at 7.58ppm. for proton at 5 position of furan ring equivalent to 1H, Triplet signal at 6.15ppm. for proton at 3 position of the furan ring equivalent to 1H. Doublet signal at 5.24ppm. for pyridine ring equivalent to 1H, quartet signal of 4.07 ppm. belongs to CH2 equivalent 2H, singlet signal at 3.43ppm. belongs to SH equivalent to 1H, Singlet signal at 2.33ppm. belongs to CH3 proton of Ethyl Ester substituent of pyridine ring equivalent to 3H triplet signal at substituent 1.12 for CH3 of ester moiety equivelent. to 3H.
Synthesis of Furyl Substituent for Naphthol Compounds (E15-19)
This series of compounds were prepared by irradiation of a mixture of β- Naphthol and one of compounds (Acet amide, urea or methyl urea and ethyl urea) in presence of Zarconyl chloride using 1,2-dichloro ethane as a solvent under Ultrasonic waves at r.t. The synthesized compounds were characterized by IR cm-1 Absorption bands: 3435-3462 related to OH stretching, 3317-3397 for NH, 1708-1747 for C=O while the aromatic C=C stretching bands appeared within range (1433-1676). The C-O-C sym. and asym. stretching bands appeared at 1020-1279 as shown in Table 7.
Furyl Substituent of α-Naphthol Compounds (E19-22)
The synthesized compounds of this series were also characterized by the following absorption bands IR cm-1 :3402- 3485 for OH stretching , 3234-3356 for NH , the carbonyl group was absorbed at 1698-1739 while the aromatic C=C appeared within the range (1458-1670) and finally C-O-C for both sym. and asym at 1009-1244 respectively as illustrated in Table 8.
Acknowledgement
Author would like to thank the Iraqi Ministry of higher Education and Research for providing I.M. Shaban a scholarship to do a PhD. In Organic chemistry in which this work is part of this degree project.
Conclusion
We have demonstrated a simple and green method for efficient synthesis of some heterocyclic compounds containing furyl derivatives within short reaction time and a moderate yield.
References
- Chemical Land21, Cyclic aldehyde (Furfural).
- Sashikala, Ong HK (2007) Synthesis and identification of furfural from rice straw. J Trop Agric 35(1) 165-172.
- Ambalkar VU, Talib MI (2012) Synthesis of furfural from lignocellulose biomass as agricultural residues. Inter J Eng sci 1(1): 30-36.
- Punsuvon V, Vaithanomsat P, Liyama K (2008) Simultaneous production of α-cellulose and furfural from bagasse by steam explosion pretreatment. Mj Int J Sci Tech 2(1): 182-191.
- Jong WD, Marcotullio G (2010) Overview of bio refineries based on Co-production of furfural, Existing concepts and novel developments. Inter J Chem Reactor Eng 8: 3-5.
- Wankasi D, Naidoo EB (2010) Furfural production from the epicrap of wild mango (irvingia species) fruits by acid catalyzed hydrolysis. Am J Food Nutr 2(2): 47-50.
- Gebre H, Fisha K, Kindeyaand T (2016) Gebremichalsynthesis of furfural from bagasse. Inter Letter Chem 5 (7): 73-84.
- Beattie S, Heibron IM, Iraving F (1932) Dicarbocyanines. A new series of cyanine dyes. J Chem Soc 260-268.
- Golʼdfarb YL, Tarasova LD (1965) Bromination products of furfural. Russian Chem Bulletin 14(6): 1041-1042.
- Aly AA, Brown AB, El-Emary TI, Ewas AM M, Ramada M (2009) Hyrazinecarbothioamide group in the synthesis of heterocycles. Arkat USA Inc pp. 173.
- Zare A, Hasaninejad A, Rostam E, Moosavi AR Z, Merajoddin M (2010) PEG-SO3H as a new, highly efficient and homo geneous polymeric catalyst for the synthesis of acylals from aldehydes and acetic anhydride. Scientia Iranica 17(1): 24-30.
- Forsido BT (2011) Synthesis of 3-methyl–TpMo(CO) 2(5-oxPyranyl) Organometallic scaffold and its application in forming quaternary center at aring junction via an oxidative annulation-demetalation cascade. M.Sc. Thesis, Emory University.
- Baumann M, Baxendale IR, Ley SV, Nikbin N (2011) An overview of the key routes to the best selling 5- membered ring heterocyclic pharmaceuticals. Beilstein J Org Chem 7: 442-495.
- Mandalika AS (2012) “Enabling the development of furan-based biorefineries. M.Sc. Thesis, University of Wisconsin-Madison.
- Martins P, Jesus J, Santos S, Raposo LR, Roma-Rodrigues C (2015) Heterocyclic Anticancer Compounds: Recent Advances and the Paradigm Shift towards the Use of Nanomedicine’s Toolbox. Molecules 20(9): 16852-16891.
- Bruno I, DeSimone R (2010) Design, Synthesis and pharma-cological studies of structural analogues modeled on bioactive natural products. PhD., Thesis, University of Salerno.
- Al-Ajely MS, Shaban IM (2018) Synthesis of Some Heterocyclic Compounds Derived from Furfural. J Addict Resea 1: 1-6.
- Al-Ajely MS, Shaman IM (2019) Synthesis of some Pipridine, Pyrmidine and Diazipine compounds contining furyl derivatives. Arch Nanomed 1(5): 133-37.
- Al-Ajelt MS, Shaban IM (2019) Synthesis of some diazine and triaazole derivatives from furfural. Int J resea 7(4): 83-89.
- Myer B (2006) preparation of 2-amino thiazole5-carboxylic acid derivitives, US patent, international application No.PCT/US2006/030198,international publication No.WO2007/019210A1.
- Bellina F, Rossi R (2007) An efficient and inexpensive multigram synthesis of 3, 4-dibromo-and 3, 4-dichlorofuran-2 (5H)-one. Synthesis 12: 1887-1889.
- Tanna JA, Chaudhary RG, Sonkusare VN, Juneja HD (2016) CuO nanoparticles: synthesis, characterization and reusable catalyst for polyhydroquinoline derivatives under ultrasonication. J Chine Advan Mater Socie 4(2): 110-122.

 Research Article
Research Article