Abstract
Extended-spectrum beta-lactamases (ESBLs) are enzymes produced by some bacteria that confer resistance to most beta-lactam antibiotics. Production of ESBLs by Klebsiella pneumoniae and Escherichia coli isolated from respiratory tract infections was investigated by phenotypic and molecular techniques. Eighty well characterized bacterial isolates comprising of 50K. pneumoniae and 30 E. coli were subjected to antibacterial susceptibility testing by the Kibrick agar diffusion method, using commercially available antibiotic discs (Oxoid, England). Twenty of the bacterial isolates, showing multiple resistances to antibiotics, made up of 13K. pneumoniae and 7 E. coli were used for ESBL detection. ESBL phenotypic detection was carried out using combination disc method, while the molecular detection was by polymerase chain reaction (PCR) amplification of bla-TEM gene. All the 80 bacterial isolates showed variable resistance to the antibiotics used. Phenotypical investigation of the 20 bacterial isolates, with multiple resistance to antibiotics, revealed 100% prevalence of β lactamase production, while PCR detected bla-TEM gene in eight out of the 20 (40%) bacteria. ESBL production by K. pneumoniae and E. coli could lead to delay in hospital outcomes in managing diseases caused by these organisms, hence the need for adequate laboratory investigations before administration of antibiotics.
Keywords: Antibiotic resistance, β-lactamase, Klebsiella pneumoniae and Escherichia coli
Introduction
Respiratory tract is an important human system that plays a vital role in breathing processes. Respiratory tract infections are the most frequently reported of all human infections, although some of these infections are most times mild, transient lasting and sometimes self-limiting, which makes many of the infected persons tend to disregard these serious conditions [1]. Respiratory tract infections are mainly caused by bacteria and viruses, and could also be due fungal and parasitic infections. It has been reported that in the United Kingdom; approximately 12.7 million people (approximately 1 in 5) have history of respiratory illnesses. Lung diseases have been reported as one of the leading causes of death in the UK. During 2008-2012, lung diseases were responsible for 20% of all the deaths in UK each year [2]. Bacteria of the family Enterobacteriaceae are important cause of nosocomial infections, especially the predominantly active species such as E. coli and Klebsiella, and they are of public health importance. These organisms do cause pneumonia, sepsis, post-surgical and urinary tract infections [3]. Although K. pneumoniae is a normal floral of the skin, mouth and intestines, when aspirated it can cause destructive changes in the alveoli resulting in bloody sputum Ryan [4]. Klebsiella pneumonia is commonly implicated in hospital acquired urinary tract infection and chronic obstructive pulmonary disease individuals. Aspiration and alcoholism have been found to be risk factors [5]. E. coli respiratory tract infections are commonly associated with E. coli urinary tract infection.
It may also result from micro-aspiration of upper airway secretions previously colonised by this organism in severely ill-patients hence it is a cause of nosocomial pneumonia. E. coli pneumonia may also be community acquired in patients with underlying diseases such as diabetes mellitus, alcoholism, and chronic obstructive pulmonary disease. E. coli pneumonia often manifests as a bronchopneumonia of the lower lobes and may be complicated by empyema [6]. Bacteria resistance to various classes of antimicrobial agents has been reported in both human and veterinary medical practice and continues to be on the increase worldwide [7-9]. Antibiotics resistance happens when germs develop ability to defeat the drugs designed to kill them. The germs do not die and continue to grow. Antibiotic resistance often led to higher medical cost, prolonged hospital stay and increased mortality [9]. Extended-spectrum beta-lactamases (ESBLs) are major enzymes produced by some bacteria that confer resistance to most beta-lactam antibiotics, including penicillins, cephalosporins, monobactam and aztreonam [10]. β-lactamases are divided into four classes (Class A-D) based on their amino acid sequence.
Classes A, C and D function by serine ester hydrolysis mechanism, while the class B beta-lactamases have a Zinc ion participating in catalysis. Functionally β-lactamases are grouped into three groups: Group 1cephalosphorinases (Class C), Group 2 serine β-lactamases (Classes A and D), and Group 3 metallo-β-lactamases (Class B), each of which is further divided several different subgroups Ozturk et al. [11]. TEM-1 is the most commonly encountered β-lactamase in Gram-negative bacteria. Up to 90% of ampicillin resistance in E. coli is due to the production of TEM-1. Carbapenemases are a diverse group of β-lactamases that are active not only against the oxyimino-cephalosporins and cephamycins but also against the carbapenems [12]. Infections with ESBL-producing organisms have been associated with poor outcomes and thus Community and hospital-acquired ESBL-producing Enterobacteriaceae is prevalent worldwide [12]. The study was aimed at assessing the prevalence of β lactamase producing K. pneumoniae and E. coli isolates from respiratory tract infections.
Methodology
Collection of Bacterial Culture
Eighty well characterized bacterial isolates comprising of 50K. pneumoniae and 30 E. coli were collected from the stock cultures of the Microbiology Laboratory, Department of Biological Sciences, Afe Babalola University Ado Ekiti, Nigeria, and were used for this study. The bacteria were previously isolated and identified from cases of respiratory tract infections.
Re-Characterization and Identification of the Bacterial Isolates
All isolates were tested for purity and re-characterised using standard microbiological and biochemical tests as described by Barrow and Feltham [13]. The bacterial isolates were identified, based on their cultural, morphological and biochemical characteristics, with the help of online Gideon informatics [14], and with reference to Garrity et al. [15].
Antibiotics Susceptibility Test
Bacterial susceptibility to antimicrobial agents was determined by the disk diffusion method following the guidelines of Clinical and Laboratory Standards Institute (CLSI) using commercially available antibiotic disc (Oxoid, England) [16]. The antibiotic discs used in this study were ceftriaxone, CRO (30μg); ceftazidime, CAZ (30μg); cefuroxime, CXM (30μg); cefoxitin, FOX (10μg); amoxicillin/clavulanate AUG (30μg); gentamicin, GEN (10μg); levofloxacin, LE (5μg); meropenem, MEM (10μg); imipenem, IM (10μg); ciprofloxacin, CIP (5μg); cefpodoxime, CPD (10μg); ofloxacin, OFX (5μg); cefixime, CFM (5μg); ampicillin, AMP (10μg); chloramphenicol, CHL (30μg); Nitofurantoin, NIT (300μg) and tetracycline, TET (30μg). E. coli ATCC® 2592 was used as quality control organism.
Phenotypic Detection of ESBL Production
Twenty of the bacterial isolates, showing multiple resistances to antibiotics, made up of 13K. pneumoniae and 7 E. Coli, were screened for the production of ESBLs by using the disk combination test by assessing a ≥5mm increase in diameter of zone of inhibition between the antimicrobial (beta-lactam) tested in combination with clavulanic acid and the diameter when the agent is tested alone, after overnight incubation at 37 °C [16]. The antibiotics used were); ceftazidime, CAZ (30μg); cefuroxime, CXM (30μg); cefoxitin, FOX (10μg) and cefpodoxime, CPD (10μg).
Plasmid DNA Extraction
Plasmid DNA extraction from 24 hour overnight on Trypticase Soy Broth cultures of the bacteria was carried out with the aid of GeneJET Plasmid Miniprep Kit (Thermo Fisher Scientific). The procedure for plasmid extraction was of four parts namely, Lysate preparation, binding to column, washing bound DNA and elution of pure DNA; with strict adherence to manufacturer guide. The elution was then run in 1% agarose gel electrophoresis and the remaining plasmid DNA was stored at -20 °C for Polymerase chain reaction (PCR) analysis of TEM gene.
PCR Amplification of β-lactamase blaTEM Gene
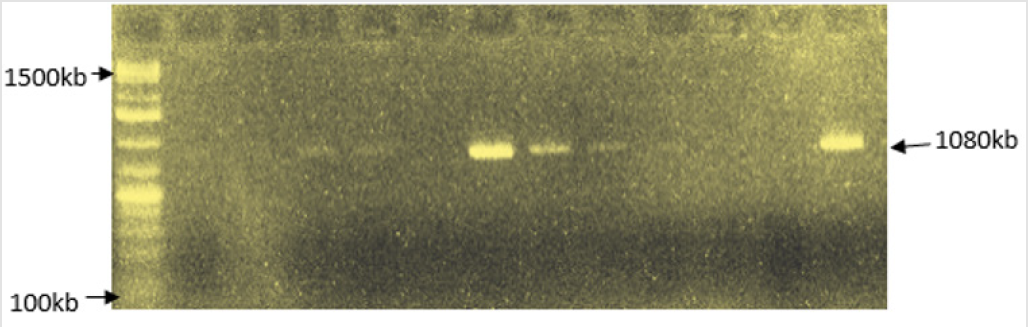
The PCR amplification of β-lactamase blaTEM gene was carried out using the forward and reverse blaTEM primers: TEM-F:5’-AT GAGTATTCAACATTTCCG-3’ and TEM-R: 5’-CCAATGCTTAATCAGTGAGG- 3’ (1080 kb) [17] (Figure 1). Procedure: 5.0μl of 2x per master mixes was dispensed into the PCR tube. 1.0μL template DNA (10pg -1ng for plasmid and 0.1-1μg for genomic DNA) and 1μL of both forward and reverse primers (2.5μM) was added to the tube, and then 1.0μL nuclease-free water was used to bring the total volume to 10μL. The PCR mixture was mixed together appropriately and gently vortexed. The PCR tubes were placed into the thermal which was programmed to carry out the following steps according to the table below: After completion of PCR, a 9μL aliquot of reaction was mixed with 3μL of loading dye (6X) and loaded onto an agarose gel for size separation of the PCR products. The gel was run at 75v for 1hr 30min. The DNA bands were then scored against the standard DNA marker band.
Figure 1: PCR amplification of βlactamase bla TEM gene in extracted plasmid DNA from K. pneumoniae and E. coli.
Results
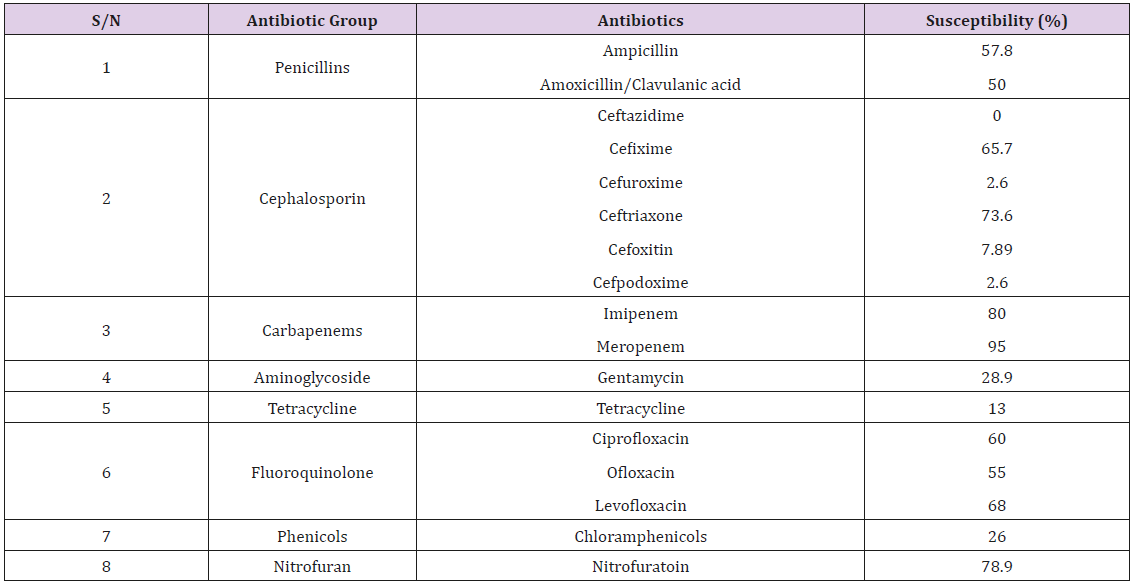
Most of the eighty bacterial isolates, made up of 50 Klebsiella pneumoniae and 30 Escherichia coli, showed resistance to at least two of the antibiotics used (Tables 1 & 2). The bacteria showed 50% and 55% susceptibility to augmentin (Amoxycillin/Clavulanic acid) and ampicillin respectively. For the cephalosporins, the bacteria were highly resistant to ceftazidime, cefuroxime, cefoxitin and ciprofloxacin; with moderate susceptibility to ceftriaxone (75.0%) and cefixime (65.7%). Gentamycin, tetracycline and chloramphenicol gave high resistance, while nitrofutantoin gave 78.9% susceptibility. Moderate susceptibilities were obtained for the fluroquinolones with ofloxacin, ciprofloxacin and levofloxacin (55, 60 and 68% respectively). High susceptibility patterns were obtained for the cabarpenes, with 85 and 95% susceptibility to imipenem and meropenem respectively.
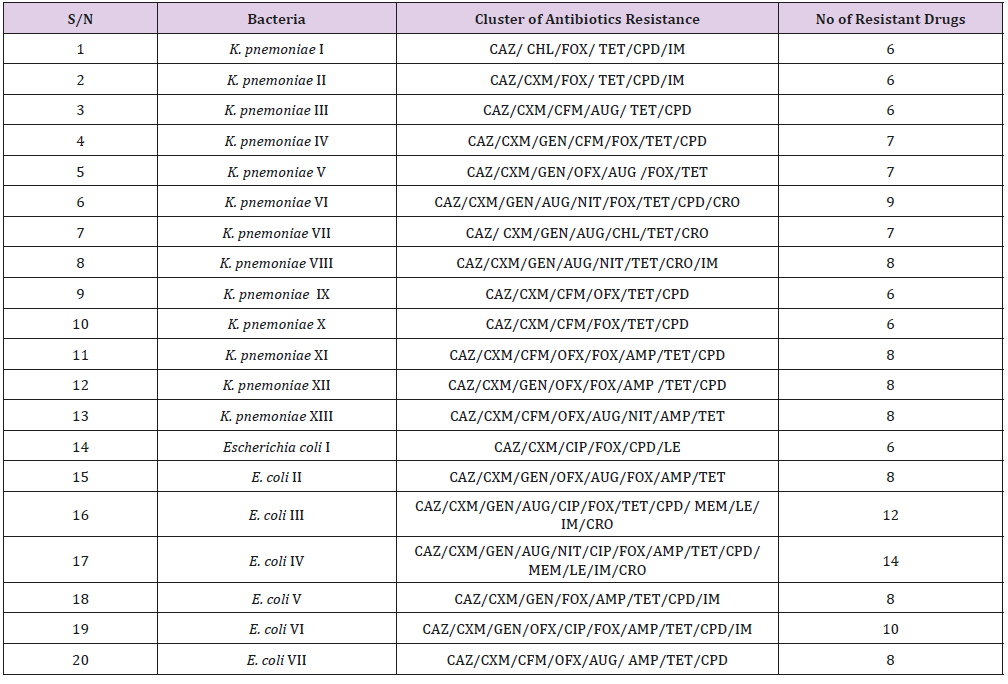
Table 2: Bacterial isolates showing multiple antibiotics resistance among K. Pnemoniae and E. coli isolates.
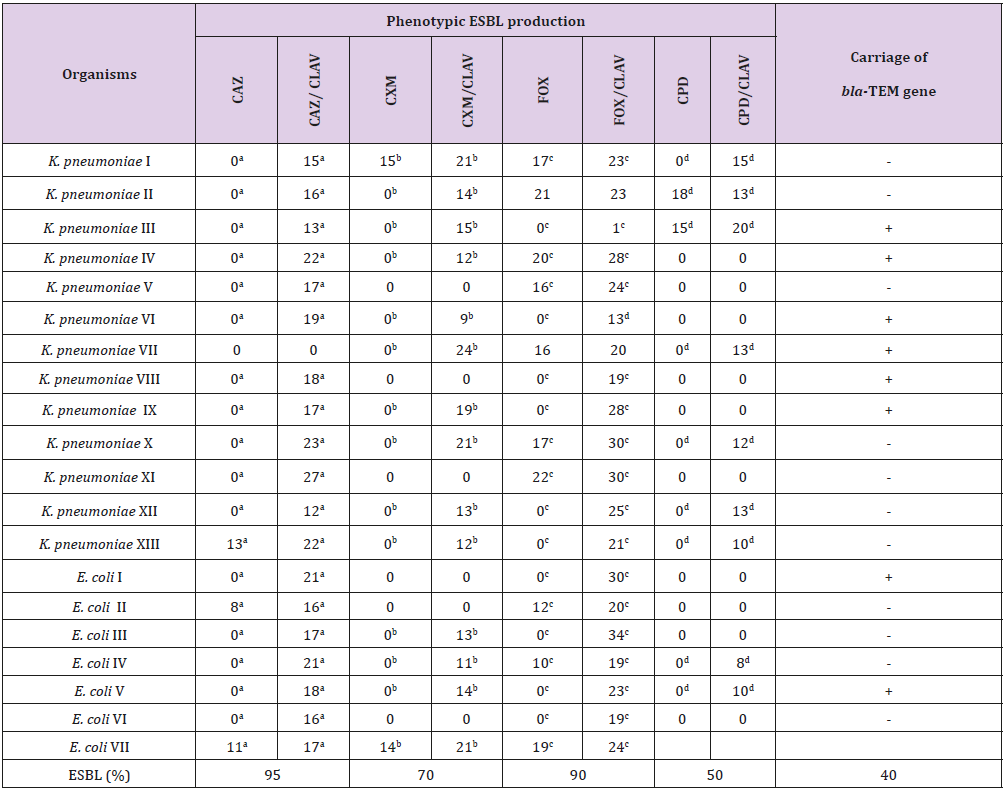
Of the twenty isolated used for the ESBL detection, the 13K. pneumoniae isolates were susceptible to meropenem, while 2 (15.4%) of the K. pneumoniae were resistant to imipenem. One (14.3%) each of the 7 E. coli isolates showed resistance to imipenem and meropenem. Resistances to cluster of 8-16 antibiotics per organism were recorded (Table 2). Phenotypical investigation revealed high prevalence (100%) of β-lactamase production by all the bacterial isolates with enhancement of zones of inhibition (≥5mm) between when the β-lactam disk was used alone and when used in combination with clavulanic acid (Table 3). The PCR amplification of bla-TEM β-lactamase gene, detected the presence of the drug resistant gene in eight (40%) out of the twenty bacterial isolates tested; 6/12 (50%) for K. pneumoniae and 2/6 (33.33%) E. coli.
Table 3: Phenotypic and Molecular characteristics of β lactamase production by K. pneumoniae and E. coli.
Note: a-fValues with same superscripts on the same row, β-lactam disk was used alone and when used in combination with clavulanic acid indicates ESBL production- Negative; + positive for blaTEM gene
Discussion
With the emergence of ESBL-producing organisms worldwide and as the number of β-lactam resistant pathogens increases, developing effective antibiotics and inhibitors for specific pathogens becomes important. The K. pneumoniae and E. coli isolates used in this study showed multiple drug resistance to as much as 14 antibiotics. This observation reiterates the finding in other studies that have reported antibiotic resistance among bacteria especially K. pneumoniae and E. coli [18,19] The high prevalence of multi-drug resistant bacteria may reflect the indiscriminate and inappropriate use of antibiotics in the study area, where these drugs are often sold over-the-counter without a physician’s prescription. All pneumoniae and E. coli isolates were highly susceptible to the carbapenems, the current drug of choice for the treatment of patients infected with multidrug-resistant ESBL-producing bacteria.
The present study reported 100% prevalence of the ESBL production among the bacterial isolates used, based on phenotypic assay, and the prevalence of 40% blaTEM gene. The blaTEM was detected in 33.33% of E. coli and 50% of K. pneumonia. The prevalence of strains of these organisms expressing the ESBL phenotype may vary across geographical regions. Bora and coworkers 19 in India had earlier reported phenotypic confirmatory of ESBL production in 73.58% of E. coli and 67.24% of K. pneumoniae; as well the prevalent genotype blaCTX-M in E. coli (88.67%) and blaTEM in K. pneumoniae (77.58%) ESBL producing isolates. They observed that majority of ESBL producing isolates were found to possess more than one ESBL genes. A recent study by researchers in Iraq, reported ESBL-producing E. coli isolates that possessed 81% blaTEM, 16.2% blaSHV, and 32.4% blaCTX-M genes; as well as 64.7% blaTEM, 35.2% blaSHV, and 41.1% blaCTX-M genes existed in the isolates of K. pneumoniae [20]. The low prevalence of genotype ESBL reported in this study may be due to low number of bacterial isolates used (due to availability of molecular kits), a higher number of isolates might have given a better result. In addition using primers of more than one beta-lactam genes will definitely increase ESBL genotype reporting among these organisms.
Conclusion
In conclusion the study showed that carbapenems followed by fluoroquinolone are the effective antibiotics against the bacterial isolates in the study area. ESBL production by K. pneumoniae and E. coli is of public health importance, as it could lead to delay in hospital outcomes in managing respiratory diseases as well as other diseases caused by these organisms.
References
- (2008) Organization WH, The global burden of disease: 2004 update.
- Yue Xianning, Liu Zhengfu, Ye Lanxian (2016) Progress in the treatment of refractory depression. Chinese Journal of Behavioral Medicine and Brain Science 25(3): 280-284.
- Zhang Y, Yeager VA, Hou S (2018) The Impact of Community-Based Supports and Services on Quality of Life Among the Elderly in China: A Longitudinal Study. J Appl Gerontol 37(10): 1244-1269.
- Beekman AT, Copeland JR, Prince MJ (1999) Review of community prevalence of depression in later life. Br J Psychiatry 174: 307-311.
- Bishwajit G, O'Leary DP, Ghosh S, Yaya S, Shangfeng T, et al. (2017) Physical inactivity and self-reported depression among middle- and older-aged population in South Asia: World health survey. BMC Geriatr 17(1): 100.
- David J (2005) Tinnitus and its American diagnosis and treatment guidelines. Chinese Journal of Hearing and Speech Rehabilitation 1: 58-61.
- Shargorodsky J, Curhan GC, Farwell WR (2010) Prevalence and characteristics of tinnitus among US adults. Am J Med 123(8): 711-718.
- Levine RA, Oron Y (2015) Tinnitus. Handb Clin Neurol 129: 409-431.
- Adoga AA, Kokong DD, Nimkur TL, Okwori ET (2015) The impact of tinnitus on adult Nigerians: health related Quality of Life assessment of sufferers using the Hospital Anxiety and Depression Scale (HADS) and the RAND-36 item health survey 1.0 questionnaire. Int Tinnitus J 19(2): 26-32.
- Aazh H, Landgrebe M, Danesh AA (2019) Parental Mental Illness in Childhood as a Risk Factor for Suicidal and Self-Harm Ideations in Adults Seeking Help for Tinnitus and/or Hyperacusis. Am J Audiol 28(3): 527-533.
- Ziai K, Moshtaghi O, Mahboubi H, Hamid R Djalilian (2017) Tinnitus Patients Suffering from Anxiety and Depression: A Review. Int Tinnitus J 21(1): 68-73.
- Kehrle HM, Sampaio AL, Granjeiro RC, De Oliveira TS, Oliveira CA (2016) Tinnitus Annoyance in Normal-Hearing Individuals: Correlation With Depression and Anxiety. Ann Otol Rhinol Laryngol 125(3): 185-194.
- Sindhusake D, Mitchell P, Newall P, Golding M, Rochtchina E, et al. (2003) Prevalence and characteristics of tinnitus in older adults: the Blue Mountains Hearing Study. Int J Audiol 42(5): 289-294.
- Langguth B, Landgrebe M, Kleinjung T, Sand GP, Hajak G (2011) Tinnitus and depression. World J Biol Psychiatry 12(7): 489-500.
- Birgit M, Heidemarie H, Heidi O, Agnieszka J Szczepek (2012) Stress and tinnitus-from bedside to bench and back. Front Syst Neurosci 6: 47.
- Eggermont JJ, Roberts LE (2015) Tinnitus: animal models and findings in humans. Cell Tissue Res 361(1): 311-336.
- Mc Cormack A, Edmondson Jones M, Fortnum H, Dawes PD, Middleton H, et al. (2015) Investigating the association between tinnitus severity and symptoms of depression and anxiety, while controlling for neuroticism, in a large middle-aged UK population. Int J Audiol 54(9): 599-604.
- Bhatt JM, Bhattacharyya N, Lin HW (2017) Relationships between tinnitus and the prevalence of anxiety and depression. Laryngoscope 127(2): 466-469.
- Li Wei, Wang Mingxi, Zhou Wei (2019) Study on anxiety and depression status and related factors in patients with idiopathic tinnitus. Journal of Clinical Otorhinolaryngology Head and Neck Surgery 33(5): 37-42.
- Chen YC, W Xia, Chen H, Feng Y, Xu JJ, et al. (2017) Tinnitus distress is linked to enhanced resting-state functional connectivity from the limbic system to the auditory cortex. Hum Brain Mapp 38(5): 2384-2397.
- Besteher B, Gaser C, Ivansic D, Orlando Guntinas Lichius, Christian Dobel, et al. (2019) Chronic tinnitus and the limbic system: Reappraising brain structural effects of distress and affective symptoms. Neuroimage Clin 24: 101976.
- Sun W, Lu J, Stolzberg D, Gray L, Deng A, et al. (2009) Salicylate increases the gain of the central auditory system. Neuroscience 159(1): 325-334.
- Ménard C, Hodes GE, Russo SJ (2016) Pathogenesis of depression: Insights from human and rodent studies. Neuroscience 321: 138-162.
- Rauschecker JP, May ES, Maudoux A, Ploner M (2015) Frontostriatal Gating of Tinnitus and Chronic Pain. Trends Cogn Sci 19(10): 567-578.
- Lai Renzhen, Ma Xin (2017) Switch to Hearing Loss and Tinnitus--A Control Gate. Journal of Clinical Otorhinolaryngology Head and Neck Surgery 31(7): 6-8.
- PANG Ying, ZENG Ji Hong, KANG Hou Xi (2018) Research progress in the pathogenesis of tinnitus. International Journal of Otorhinolaryngology Head and Neck Surgery 42(6): 330-334.
- Coskunoglu A, Orenay Boyacioglu S, Deveci A, Bayam M, Onur E, et al. (2017) Evidence of associations between brain-derived neurotrophic factor (BDNF) serum levels and gene polymorphisms with tinnitus. Noise Health19(88): 140-148.
- Zhang Jianping, Cao Tingting, Ren Bingqi (2018) Characteristics of cognitive impairment of chronic subjective tinnitus. Journal of Apoplexy and Nervous Diseases 35(12): 57-60.
- Lee YJ, Bae SJ, Lee SH, Lee JJ, Lee KY, et al. (2007) Evaluation of white matter structures in patients with tinnitus using diffusion tensor imaging. J Clin Neurosci 14(6): 515-519.
- Leaver AM, Seydell Greenwald A, Turesky TK, Susan Morgan, Hung J Kim et al. (2012) Cortico-limbic morphology separates tinnitus from tinnitus distress. Front Syst Neurosci 6: 21.

 Research Article
Research Article