Abstract
The kinetic laws of the condensation reaction between metal acetates are investigated: - manganese and copper Me[OC(O)CH3]2 (Me(Ac)2) with N-methylolurea. The numerical values of the rate constant at various temperatures and the activation energy of the condensation process between these compounds are estimated. A conclusion is drawn regarding the activity of the acetates of the studied metals in the reactions of their interaction with N-methylolurea.
Keywords: N-Methylolurea; Manganese and Copper Acetates; Rate Constant; Activation Energy
Introduction
The synthesis and study of the properties of alcoholates of metals of variable valence has been the subject of many works, of which the authors’ work deserves special attention [1-3]. Alcoholates of metals of variable valency are interesting in that they exhibit catalytic activity both in the synthesis of individual organic compounds and in the complex radical polymerisation of vinyl monomers [4,5]. So, in the aforementioned work [4], in particular, a high catalytic activity of metal alcoholates-manganese and copperwas noted. Meanwhile, in the scientific literature there is practically no data on a quantitative assessment of the formation of alcoholates from acetates of the above metals. This report is devoted to a detailed study of the reaction between metal acetates: -manganese and copper with N-methylurea at various temperatures, followed by determination of both the rate constant and the activation energy of their interaction.
Experimental Part
Mn [OC(O)CH3]2 ∙ 4H2O and Cu[OC(O)CH3]2 ∙ H2O were used in the studies “chda” brand, N - methylolurea was synthesized according to the method described in detail in [6] and identified by IR spectroscopy on a NIKOLET / FT-IRNEXUS spectrophotometer. Getting MMch. 15 g (0.25 mol) of urea and 20 g of a 38% aqueous formaldehyde solution (0.25 mol) are loaded into the reactor and stirred at a temperature of (35 ± 0.5) 0С for 30 minutes. Then under low pressure (15-20) mm. Hg. Art. at a temperature of (45-50)0С water is distilled off. The white precipitate (MMh) is repeatedly washed and recrystallized with ethanol and dried under pressure (15-20) mm Hg. at 50-600С until a constant mass is achieved. The yield of MMh is 93%, the melting point (Tm) is (111 ± 0.5)0С. The elemental composition in (%) was established: C- 26.6(26.67); H6.8(6.66); N-31.2(31.11). In our studies, a mixture of dimethylformamide (DMF) with water in a volume ratio (1:1) was used as an MMh solvent. Acetic acid obtained during the reaction was determined by volumetric analysis i.e. titration of 4 ml of the sample with 0.1 N aqueous alkali solution (NaOH) prepared from fixanal. The indicator was phenophthalein. The total volume of the reaction mixture was 50 ml.
The Discussion of The Results
The kinetic regularities of the accumulation of acetic acid formed in the reaction between Mn [OC(O)CH3]2(Mn (Ac)2) and N-methylolurea (MMh) at various temperatures studied, depending on the reaction time, are given in Table 1. From the data in Table 1 it follows that the reaction between Mn (Ac)2 and MMh is a second-order reaction, and the rate constant is described [7] by the equation below

Using the data in Table 1 and plotting the dependencies
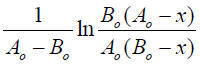 from t (reaction time) are estimated and presented
in Table 2 values of the reaction rate constant between Mn (Ac)2 and
MMh at different temperatures. Induction periods shown in Figure
1 regularities can be attributed to the lifetime of the intermediate
compound formed upon the interaction of MMh with Mn (Ac)2. The
probable structure of the intermediate compound and its further
decay can be represented as:
from t (reaction time) are estimated and presented
in Table 2 values of the reaction rate constant between Mn (Ac)2 and
MMh at different temperatures. Induction periods shown in Figure
1 regularities can be attributed to the lifetime of the intermediate
compound formed upon the interaction of MMh with Mn (Ac)2. The
probable structure of the intermediate compound and its further
decay can be represented as:
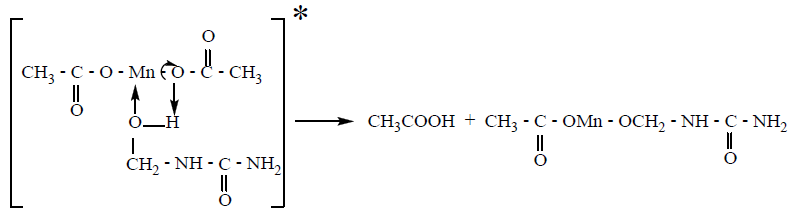
Figure 1: Dependence 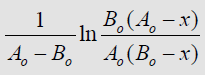 from t (min) at various temperatures: 1- (600С); 2- (650С); 3- (700С); 4- (750С).
from t (min) at various temperatures: 1- (600С); 2- (650С); 3- (700С); 4- (750С).
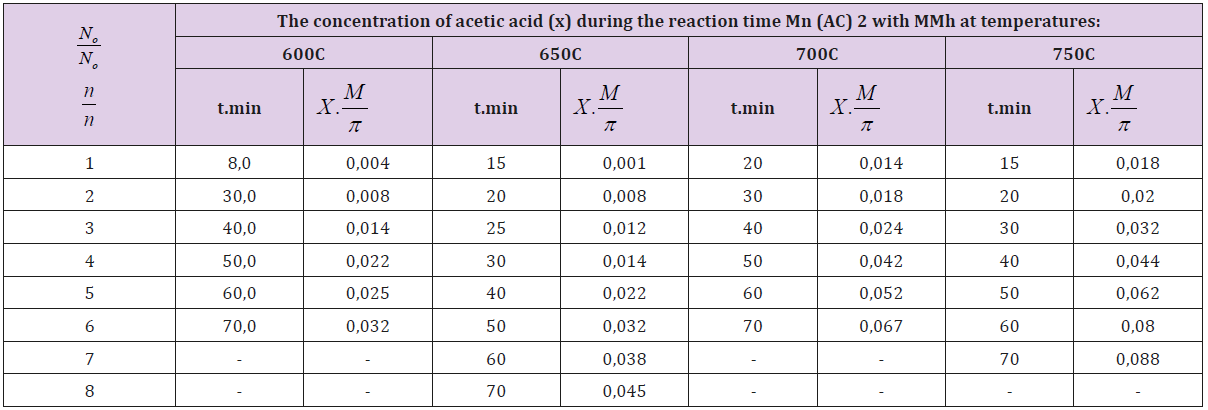
Table 1: Kinetics of the reaction between Mn (Ac)2 and MMh at different temperatures [MMh]0 = (A)0=0.6 mol / l; [Mn (Ac)2] 0 =(V) 0-0.3 mol/L.
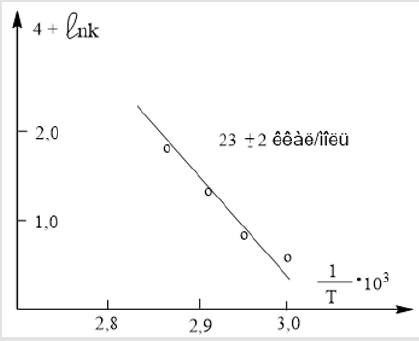
Using the Arrhenius equation
Ea 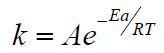 (2) [7] and
constructing the dependence of ln
(2) [7] and
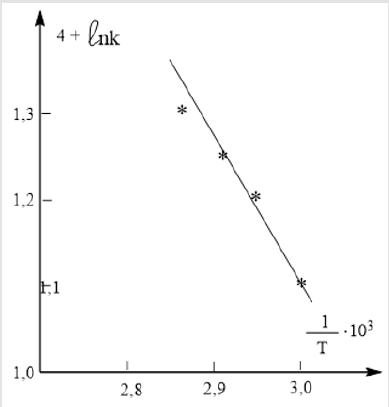
constructing the dependence of ln 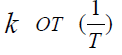 (Figure 2) based on
the numerical values of k of (Table 2), the activation energy (Еа )
the reaction of the interaction of MMC with Mn (Ac)2, which turned
out to be equal to (23±2) kcal / mol. The data in Table 3 indicate
that the reaction between Cu (Ac)2 and MMh, like the reaction of
manganese acetate Mn (Ac)2 with MMh, is a second-order reaction,
and the rate constant is described by equation (1). Using the data
in (Table 3) and plotting the dependencies
(Figure 2) based on
the numerical values of k of (Table 2), the activation energy (Еа )
the reaction of the interaction of MMC with Mn (Ac)2, which turned
out to be equal to (23±2) kcal / mol. The data in Table 3 indicate
that the reaction between Cu (Ac)2 and MMh, like the reaction of
manganese acetate Mn (Ac)2 with MMh, is a second-order reaction,
and the rate constant is described by equation (1). Using the data
in (Table 3) and plotting the dependencies 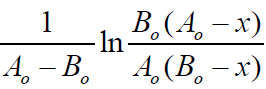 from t
(reaction time) are estimated and presented in (Table 4) values of
the reaction rate constant between Cu (Ac)2 and MMh at different
temperatures (Figure 3). Yeritsyan M.L. Using the data of Table 4 and plotting the dependence of ln
from t
(reaction time) are estimated and presented in (Table 4) values of
the reaction rate constant between Cu (Ac)2 and MMh at different
temperatures (Figure 3). Yeritsyan M.L. Using the data of Table 4 and plotting the dependence of ln 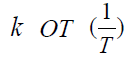 (Figure 4), the value
of the activation energy of the reaction between Cu (Ac)2 and MMh
was estimated, which turned out to be 10 ± 1.5 kcal / mol. Thus,
from a comparison of the numerical values of the interaction
activation energy, we can conclude that copper acetate (Cu (Ac)2)
in the reaction with MMh is more active than manganese acetate
(Mn (Ac)2).
(Figure 4), the value
of the activation energy of the reaction between Cu (Ac)2 and MMh
was estimated, which turned out to be 10 ± 1.5 kcal / mol. Thus,
from a comparison of the numerical values of the interaction
activation energy, we can conclude that copper acetate (Cu (Ac)2)
in the reaction with MMh is more active than manganese acetate
(Mn (Ac)2).
Figure 3: Dependence 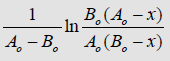 from OT t (min) at various temperatures: 1- (600С); 2-(650С); 3- (700С); 4- (750С)..
from OT t (min) at various temperatures: 1- (600С); 2-(650С); 3- (700С); 4- (750С)..
Table 3: The change in the concentration of acetic acid over the course of the reaction between [Cu (Ac)2] and (MMh) at various temperatures studied: [MMh]0 = (A) 0 = 0.6 mol / l; [Cu(Ac)2] 0 = (V) 0 = 0.3 mol/L.
References
- ML Yeritsyan, RR Karapetyan, KA Martirosyan, RA Karamyan, AG Khachatryan, et al. (2011) Scientific notes of YSU, chemistry and biology 3: 16.
- ML Yeritsyan, SM Petrosyan, RA Karamyan, LM Yeritsyan (2014) Physicochemistry of polymers, synthesis, properties and applications. Tver Bulletin of TSU 20: 284.
- RA Qaramyan, IN Sirekanyan, ML Yeritsyan (2018) Amino alcohols and chelates on their basic.- Proceedings of the Yerevan State Universiti. chemical and biological Sciences 52(8): 96.
- NV Smirnov, GA Gabrielyan L, S Holbraich (1990) High molecular weight compound 32(11): 2314.
- VA Kabanov, VP Teeth, Yu D Semchikov (1987) Complex radical polymerization pp. 256.
- AF Nikolaev (1964) Synthetic polymers and plastics based on them. Publishing house “Chemistry”. 378.
- NM Emanuel, DG Knorre (1962) The course of chemical kinetics. Graduate School. Moscow pp. 168.

 Research Article
Research Article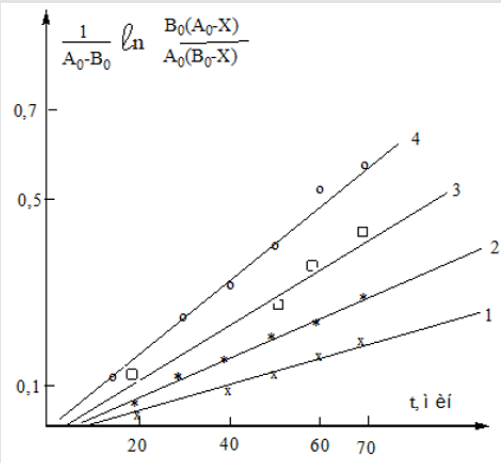

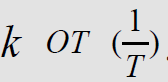 .
.
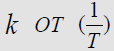 .
.