Abstract
Heart failure is with high morbidity and mortality rates. Exercise is good for heart health. Our studies used 45 male Sprague-Dawley (SD) rats weighing 150 ± 20 g that were randomly divided into a control group (normal saline, n = 15), doxorubicin (DOX) group (n = 15) and DOX plus exercise (DOX+E) group (n = 15). Heart failure was induced experimentally by intraperitoneal injections of the cardiotoxic agent DOX. All groups received different treatment for 8 weeks. Left ventricular fractional shortening and ejection fraction were higher in DOX+E group rats than in DOX group rats on an echocardiographic examination. Significant cardiac tissue damage (haematoxylin and eosin, Masson’s trichrome staining) were seen in rats of the DOX group compared to those of the DOX+E group. Exercise inhibited DOX-induced cardiac fibrosis (transforming growth factor-β, α-smooth muscle actin, collagen I, and collagen III) and oxidative stress (Nox4, p53) in an immunoblotting analysis. These results indicate that exercise may be a potential therapeutic method for cardiac damage induced by DOX-induced heart failure.
Keywords: Heart Failure; Doxorubicin; Exercise; Fibrosis; Sd Rat
Introduction
Heart Failure (HF) is an important public health problem as a result of its high morbidity and poor prognosis [1]. The clinical features of HF are decreased exercise ability and with early fatigue and dyspnea [2,3]. Myocardial fibrosis plays an important role in HF, because it mediates the process of maladaptive remodeling [4]. Myofibroblasts express α-smooth muscle actin (α-SMA), indicating the acquisition of a secretory and contractile phenotype, which are associated with increased synthesis and deposition of Extracellular Matrix (ECM) proteins including collagen [5]. Transforming growth factor-β (TGF-β) is the most effective stimulator of myocardial fibrosis, and myocardial TGF-β levels are elevated in HF. TGF-β stimulates cardiac fibroblast-myofibroblast transformation and ECM deposition [6]. Oxidative stress is considered to play a key role in the development and progression of HF-related cardiac remodeling [7]. Reactive Oxygen Species (ROS) regulate ECM remodeling according to mediating cardiac fibroblast function and stimulating collagen turnover [8]. NADPH oxidase (Nox) is the main source of ROS in the heart [9]. Nox2 and Nox4 are the primary isoforms and which expressed in the myocardium [10]. Nox4 has constitutive activity and is located in mitochondria, which is the main source of superoxide generation in the heart [11]. Current guidelines recommend regular physical exercise for patients with stable HF to prevent and/or reduce cardiac remodelling [12]. Some clinical experiment studies have shown that physical exercise can alleviate abnormal cardiac remodeling, improve blood flow and oxygen utilization, and increase cardiac function, exercise duration and quality of life [13-15]. Exercise training can reduce the risk of HF [16] and improve cardiac function [17,18].
This study used exercise training consisting of open and free swimming in warm water at a suitable depth in a swimming pool to avoid an excessive fear of drowning and struggling. The effects of this swimming exercise training on doxorubicin (DOX)-induced cardiac fibrosis and oxidative stress in the rat heart were then investigated.
Materials and Methods
The animal experimental protocols were approved by the Dalian University of Chinese Medicine Committee on Laboratory Animal Care, and all animals received humane care according to Dalian University guidelines. The Sprague-Dawley (SD) rats were provided from the Dalian Medical University Laboratory Animal Center. All animals were fed under daylight and night conditions, and they were fed and drank freely. Establishment of animal models based on previous methods [19,20]. 45 male SD rats weighing 150 ± 20 g were randomly divided into a control group (n = 15), DOX group (n = 15), and DOX plus exercise (DOX+E) group (n = 15). Within 2 weeks, HF was induced by intraperitoneal injection of cardiotoxic agent DOX at a cumulative dose of 15 mg/kg for 6 injections (DOX 2.5 mg/kg × 6). After DOX injection, swimming exercise lasted for 8 weeks.
One week before DOX injection, exercise was domesticated in the experimental swimming pool (temperature 30℃; water depth 44 cm; radius 120 cm). The progressive program initially involved swimming for 5-10 minutes and gradually extended to 50 minutes/ day. HF was induced after swimming training. When DOX was injected, rats were given formal swimming exercise for 60 minutes/ day, 5 days/week for 8 weeks. All groups of rats were anesthetized. Chest hair was removed by shaving and depilating ointment. The LV function was assessed by echocardiography using a high-resolution small animal imaging system, in which the animals were placed on a heating platform in a horizontal position. Two-dimensional and M-mode echocardiographic studies were performed from shortaxis view and end-systolic and ventricular dimensions [21]. Left ventricular (LV) function was assessed by ejection fraction (EF), shortened fraction (FS) and end-diastolic and systolic diameter of LV.
Cardiac tissues were immobilized with 10% buffered formalin solution for 30 minutes, then dehydrated overnight in 75% ethanol, followed by paraffin embedding. Continuous 4-micron sections were cut. Sections were stained with hematoxylin and eosin (HE) for histological analysis. Rat cardiac tissues from each group were stored in 10% formalin for 2 weeks, dehydrated in a rising series of alcohols (75%, 85%, 90% and 100% alcohols, each for 5 minutes) and embedded in paraffin. Paraffin sections 4 microns thick were cut from these paraffin-embedded tissues. Tissue sections were deparaffinized by immersing xylene (three times, five minutes each) and hydrated with a series of alcohols (100%, 90%, 85% and 75% alcohols, five minutes each). Masson trichrome staining was used to stain the biopsy samples to study the changes of cardiac morphology and fibrosis. Blue staining represents the accumulation of collagen. The results were observed by Olympus B X 40 upright light microscope.
Proteins were extracted from cardiac tissues using radio immunoprecipitation assay buffer. Samples were electrophoresed on 10% SDS-PAGE gel, and proteins were transferred to polyvinylidene fluoride membrane. Membranes were blocked in Tris-buffered saline with 0.1% Tween-20 (TBS-T) containing 5% skim milk, and then were incubated in primary antibody diluent and gently shaken overnight at 4°C. Primary antibodies against TGF-β (rabbit anti-TGF-β antibody, 1:1000; Proteintech, Wuhan, China), α-SMA (rabbit anti-α-SMA antibody, 1:1000; Proteintech), collagen I (rabbit anti-collagen I antibody, 1:1000; Proteintech), collagen III (rabbit anti-collagen III antibody, 1:1000; Proteintech), Nox4 (rabbit anti-Nox4 antibody, 1:1000; Proteintech), p53 (rabbit anti-p53 antibody, 1:1000; Proteintech), and anti-β-actin (1:1000; Cell Signaling Technology, USA). After washing, membranes were then incubated with secondary antibody (anti-rabbit Ig-G, 1:1000; Cell Signaling Technology) for 1 hour. This analysis was carried out independently three times. Protein levels are expressed as protein/ β-actin ratios to minimize loading differences. The relative signal intensity was quantified using NIH ImageJ software.
All data are presented as mean ± SEM. The statistical analysis was performed using SPSS software version 23.0 (SPSS Inc., Chicago, IL, USA). Inter-group variation was measured by one-way analysis of variance and a subsequent Tukey’s test. The minimal level for significance was P < 0.05.
Results
Metabolic Characterization
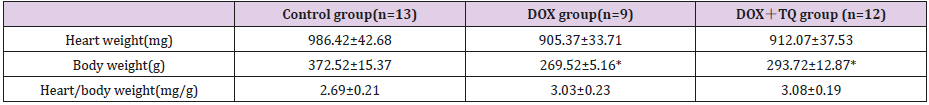
All groups SD rats after 8 weeks of different treatments, metabolic characteristics were summarized in Table 1. There was no difference in heart weight, body weight and heart/body weight among the three groups.
Table 1: Metabolic characteristics of three group rats with 8 weeks treatment.
Note: Data are given as the means ± SEM; n=9-13 in each group. * P<0.05 vs Control group.
Exercise Suppressed DOX-Induced LV Function Damage
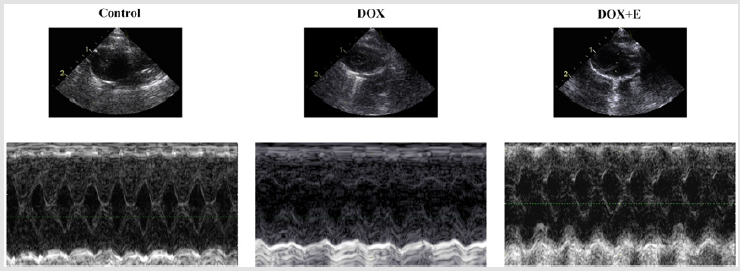
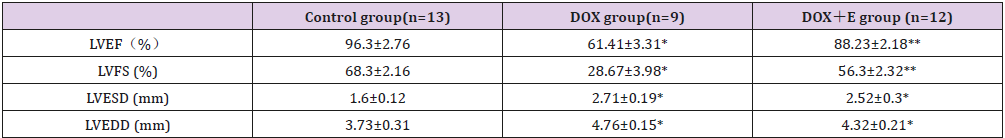
We used M-mode echocardiography to assess cardiac dimensions. Compared with the control group, LVEF and LVFS in the DOX group decreased significantly; however, with exercise, the decrease in the DOX group increased significantly. Compared with the control group, LVESD and LVEDD in DOX and DOX + E groups increased significantly, but there was no significant difference between DOX and DOX + E groups (Figure 1 and Table 2). The results suggest that exercise can protect LV function in the presence of DOX-induced cardiac injury.
Figure 1: Exercise suppressed doxorubicin-induced left ventricular function damage.
Note: Representative M-mode echocardiographic tracings.
Table 2: Echocardiographic characteristics of three group rats with 8 weeks treatment.
Note: LVEF= left ventricular ejection fraction; LVFS=left ventricular fractional shortening; LVEDD=left ventricular end-diastolic dimension; LVESD=left ventricular end-systolic dimension.
Data are given as the means ± SEM; n=9-13 in each group. * P<0.05 vs Control group; ** P<0.05 vs DOX group.
Exercise Reduced Histopathological Changes in the Cardiac Tissues
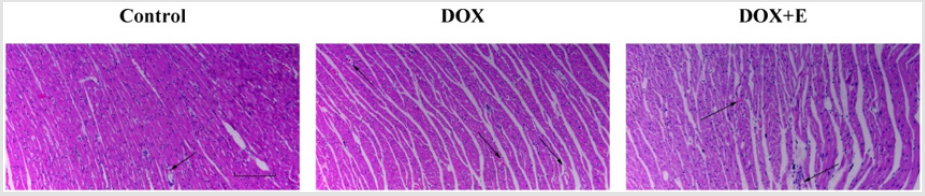
HE is staining showed that the histopathological changes in DOX group were significantly higher than those in control group: some tissues were enlarged, degenerated or dissolved, or included necrotic cardiomyocytes, myofibrillar distortion, inflammatory cell infiltration or myocardial fiber rupture. Most lesions were absorbed in DOX + E group; some patients still had a small amount of inflammatory cell infiltration (Figure 2).
Figure 2: Effect of exercise on doxorubicin-induced histopathological changes in the cardiac tissues. The histopathological changes were evaluated by haematoxylin and eosin staining (n=5). Scale bar = 200 μm. Arrows indicate positively stained cells.
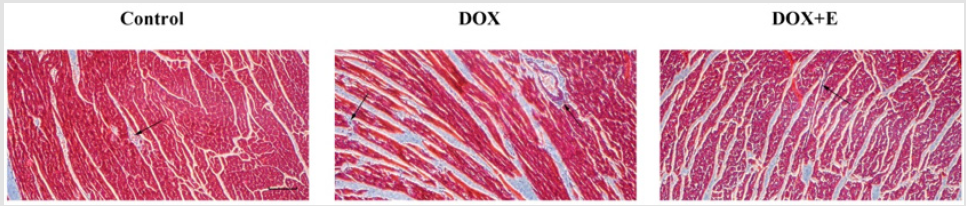
Exercise Inhibited DOX-Induced Myocardial Collagen Deposition
To assess LV collagen deposition, Masson’s trichrome staining was used to stain cardiomyocytes red and collagen fibers blue (Figure 3). Compared with the control group, DOX group showed extensive fibrous tissue in the interstitial and perivascular areas; however, rats treated with exercise after DOX-induced cardiac injury showed considerably fewer fibrous tissue, suggesting that exercise can attenuate myocardial fibrosis.
Figure 3: Effect of exercise on doxorubicin-induced left ventricular myocardial fibrosis. Fibrosis was evaluated by Masson’s trichrome staining (n=5), after which myocardial cells stained red and collagenous fibres stained blue. Scale bar = 200 μm. Arrows indicate positively stained cells.
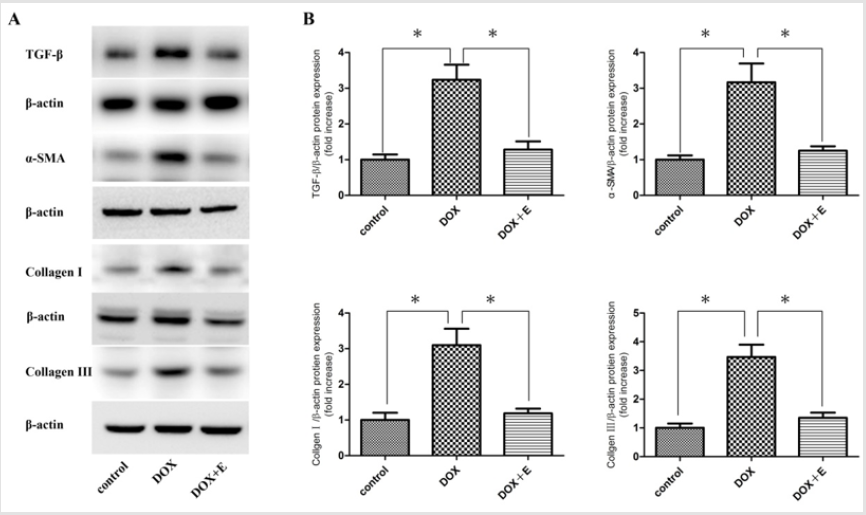
Exercise Inhibited DOX-Induced Cardiac Fibrosis
In order to study the potential mechanism of exercise on antifibrosis, the protein levels of TGF-β, α-SMA, collagen I and collagen III in cardiac tissue were determined by Western blotting. Our results showed that the levels of TGF-β, α-SMA, collagen I and collagen III in DOX + E group were significantly inhibited compared with those in DOX group (Figure 4).
Figure 4: To evaluate the effect of exercise on cardiac fibrosis. Protein levels of TGF-β, α-SMA, collagen I, and collagen III in the cardiac tissues were determined by western blotting (n = 3). (A) Immunoblotting for TGF-β, α-SMA, collagen I, and collagen III in the cardiac tissues. (B) Bar graph showing quantification of TGF-β, α-SMA, collagen I, and collagen III protein expression. Data are shown as mean ± SEM, n = 3 in each group. * P < 0.05 vs the DOX group.
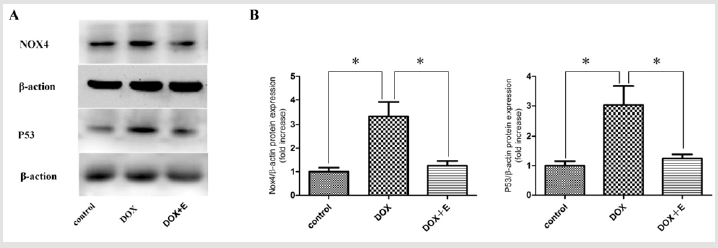
Exercise Suppressed DOX-Induced Oxidative Stress
To detect oxidative stress in myocardium, Nox4 and p53 were used for immunoblotting analysis (Figure 5). Compared with DOX group, the expression of Nox4 and p53 protein in cardiac tissue of DOX + E group was significantly lower. These results suggest that exercise reduces myocardial oxidative stress in DOX-induced rats.
Figure 5: Nox4 and p53 protein expressions in the cardiac tissues of the three groups after 8 weeks of different treatments (n = 3). Protein bands and quantification of protein levels (mean ± SEM). *P < 0.05 vs the DOX group.
Discussion
Here we showed that exercise improves the cardiac functional capacity of rats with DOX-induced HF. We also assessed the influence of physical exercise on cardiac fibrosis and oxidative stress. There was no difference in body weight among the three groups. Weight loss, known as cardiac cachexia, is common in the late stages of heart failure in humans, and is associated with poor prognosis, regardless of important variables such as age, EF, exercise capacity or functional class [22]. In our study, observed weight retention showed that rats that survived to the end of the study did not have severe HF. We performed transthoracic echocardiography and tissue Doppler imaging prior to the exercise protocol to evaluate the degree of cardiac injury induced by DOX and ensure intergroup homogeneity. Exercise may effectively increase LVEF and LVFS, leading to improved LV function in the DOX-induced rat HF model. Histomorphological damage was significant reduced in the DOX+E group compared with the DOX group as evidenced by HE is staining. Increased synthesis or decreased collagen degradation play an important role in cardiac damage after HF, which ultimately affects LV function. Our study showed that myocardial collagen deposition in the HF group was significantly increased compared with that in control group by Masson’s trichrome staining, however, with exercise treatment group showed myocardial collagen deposition was decreased.
Cardiac fibrosis is a complex process involving multiple pathways of interaction, but TGF-β plays an important role in cardiac fibrosis. Previous studies have shown that TGF-β promotes scar tissue formation during scar formation [23,24]. It also induces fibroblasts to transform into myofibroblasts [25]. Myofibroblasts are found in abnormal myocardium, characterized by the presence of microfilamentary contractile devices rich in α-SMA. Persistent myofibroblasts lead to excessive scarring and further loss of tissue compliance [25]. Many studies have shown that the expression of collagen I and collagen III in cardiac fibroblasts are significantly increased [26,27]. Our study findings are in agreement with these reports. The present results show that TGF-β, α-SMA, collagen I, and collagen III protein expressions were significantly increased in the DOX group compared to the control group; however, these increases were markedly reduced in the DOX+E group rats. These results indicate that exercise reduced cardiac fibroblasts according to TGF-β, α-SMA, collagen I, and collagen III.
Nox4 in myocardial cells is the main source of mitochondrial oxidative stress, which mediates mitochondrial and cardiac dysfunction [28]. P53 is a stress-induced transcription factor that can be activated by several adverse stimuli, including DNA damage, hypoxia and ROS. Under these conditions, increased expression of p53 protein leads to growth arrest or apoptosis [29,30]. Ying W et al. [31] reported that Nox4 and p53 play critical roles in cardiomyocyte injury. The present results showed that Nox4 and p53 protein expression significantly increased in the DOX group compared to the control group; however, exercise can markedly decrease Nox4 and p53 protein expression in DOX-induced HF rats. Exercise improves the functional capacity of rats with DOX-induced HF regardless of changes in cardiac structures, LV function, cardiac fibrosis, and oxidative stress. These findings provide new insight into the role of exercise in DOX-induced HF and identify a novel potential therapeutic intervention to treat HF.
Acknowledgement
This work was finally supported by The Doctoral Scientific Research Foundation of Zhongshan Hospital Affiliated to Dalian University (DLDXZSYY-BK201804).
References
- Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, et al. (2015) Heart disease and stroke statistics-2015 update: a report from the American Heart Association. Circulation 131(4): 29-322.
- Mandic S, Myers J, Selig SE, Levinger I (2012) Resistance versus aerobic exercise training in chronic heart failure. Current Heart Failure Reports 1: 57-64.
- Phillips SA, Vuckovic K, Cahalin LP, Baynard T (2015) Defining the system: contributors to exercise limitations in heart failure. Heart Failure Clinics 1: 1-16.
- Petrov VV, Fagard RH, Lijnen PJ (2002) Stimulation of collagen production by transforming growth factor-beta1 during differentiation of cardiac fibroblasts to myofibroblasts. Hypertension 39: 258-263.
- Porter KE, Turner NA (2009) Cardiac fibroblasts: at the heart of myocardial remodeling. Pharmacol Ther 123: 255-278.
- Brown RD, Ambler SK, Mitchell MD, Long CS (2005) The cardiac fibroblast: therapeutic target in myocardial remodeling and failure. Annu Rev Pharmacol Toxicol 45: 657-687.
- Kinugawa S, Tsutsui H, Hayashidani S, Ide T, Suematsu N, Satoh S, et al. (2000) Treatment with dimethylthiourea prevents left ventricular remodeling and failure after experimental myocardial infarction in mice: role of oxidative stress. Circ Res 87: 392-398.
- Murdoch CE, Zhang M, Cave AC, Shah AM (2006) NADPH oxidase-dependent redox signalling in cardiac hypertrophy, remodelling and failure. Cardiovasc Res 71: 208-215.
- Octavia Y, Brunner-La Rocca HP, Moens AL (2012) NADPH oxidase-dependent oxidative stress in the failing heart: From pathogenic roles to therapeutic approach. Free Radic Biol Med 52: 291-297.
- Byrne JA, Grieve DJ, Bendall JK, Li JM, Gove C, et al. (2003) Contrasting roles of NADPH oxidase isoforms in pressure-overload versus angiotensin II-induced cardiac hypertrophy. Circ Res 93: 802-805.
- McMurray JJV, Adamopoulos S, Anker SD (2012) ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. European Heart Journal 14: 1787-1847.
- Flynn KE, Piña IL, Whellan DJ, Lin L, Blumenthal JA, et al. (2009) Effects of exercise training on health status in patients with chronic heart failure. The Journal of the American Medical Association.2009 14: 1451-1459.
- Negrao CE, Middlekauff HR, Gomes Santos IL, Antunes Correa LM (2015) Effects of exercise training on neurovascular control and skeletal myopathy in systolic heart failure. The American Journal of Physiology—Heart and Circulatory Physiology 8: 792-802.
- Campos JC, Fernandes T, Bechara LR (2015) Increased clearance of reactive aldehydes and damaged proteins in hypertension-induced compensated cardiac hypertrophy: impact of exercise training. Oxidative Medicine and Cellular Longevity p. 11.
- Whellan DJ, Kraus WE, Kitzman DW, Rooney B, Keteyian SJ, et al. (2015) Authorship in a multicenter clinical trial: The Heart Failure-A Controlled Trial Investigating Outcomes of Exercise Training (HF-ACTION) Authorship and Publication (HAP) scoring system results. American heart journal 169: 457-463.
- Wang M and Shah AM (2015) Age-associated pro-inflammatory remodeling and functional phenotype in the heart and large arteries. Journal of molecular and cellular cardiology 83: 101-111.
- Asrar Ul Haq M, Goh CY, Levinger I, Wong C and Hare DL (2015) Clinical utility of exercise training in heart failure with reduced and preserved ejection fraction. Clinical Medicine Insights Cardiology 9: 1-9.
- Lee PJ, Rudenko D, Kuliszewski MA, Liao C, Kabir MG, et al. (2014) Survivin gene therapy attenuates left ventricular systolic dysfunction in doxorubicin cardiomyopathy by reducing apoptosis and fibrosis. Cardiovasc Res 3: 423-433.
- Ammar HI, Saba S, Ammar RI, Elsayed LA, Ghaly WB, Dhingra S (2011) Erythropoietin protects against doxorubicin-induced heart failure. Am J Physiol Heart Circ Physiol 6: 2413-2421.
- Condorelli G, Morisco C, Stassi G, Notte A, Farina F, et al. (1999) Increased cardiomyocyte apoptosis and changes in proapoptotic and antiapoptotic genes bax and bcl-2 during Left ventricular adaptations to chronic pressure overload in the rat. Circulation 99: 3071-3078.
- Anker SD, Negassa A, Coats AJS (2003) Prognostic importance of weight loss in chronic heart failure and the effect of treatment with angiotensin-converting-enzyme inhibitors: an observational study. The Lancet 363: 1077-1083.
- Park JY, Ryu SK, Choi JW, Ho KM, Jun JH (2014) Association of inflammation, myocardial fibrosis and cardiac remodelling in patients with mild aortic stenosis as assessed by biomarkers and echocardiography. Clin Exp Pharmacol Physiol 41:185-191.
- Yang X, Zhang H, Jia Y, Ni L, Li G, et al. (2013) Effects of intermedin1-53 on myocardial fibrosis. Acta Biochim Biophys Sin 45: 141-148.
- Lal H, Ahmad F, Zhou J, Yu JE, Vagnozzi RJ, et al. (2014) Cardiac Fibroblast Glycogen Synthase Kinase-3β Regulates Ventricular Remodeling and Dysfunction in Ischemic Heart. Circulation 130: 419-430.
- Chen X, Xu J, Jiang B, Liu D (2006) Bone morphogenetic protein-7 antagonizes myocardial fibrosis induced by atrial fibrillation by restraining transforming growth factor-β (TGF-β)/Smads signaling. Med Sci Monit 22: 3457-3468.
- Guo Y, Dong Z, Shi Y, Wang W, Wang L, et al. (2016) Sonodynamic Therapy Inhibits Fibrogenesis in Rat Cardiac Fibroblasts Induced by TGF-β Cell Physiol Biochem 40: 579-588.
- Kuroda, J, Sadoshima, J (2010) NADPH oxidase 4 (Nox4) is a major source of oxidative stress in the failing heart. PNAS 107: 15565-15570.
- Lowe SW, Schmitt EM, Smith SW, Osborne BA, Jacks T (1993) P53 is required for radiation-induced apoptosis in mouse thymocytes. Nature.1993 362: 847-849.
- Polyak K, Xia Y, Zweier JL, Kinzler KW, Vogelstein B (1997) A model for p53-induced apoptosis. Nature 389: 300-305.
- Wang Y, Zhong L, Liu X, Zhu YZ (2017) ZYZ-772 Prevents Cardiomyocyte Injury by Suppressing Nox4-Derived ROS Production and Apoptosis. Molecules 22(2).

 Research Article
Research Article