Abstract
Fertility preservation has been traditionally recommended for cancer and infertile patients. However, it is now increasingly utilized by healthy men and women due to personal preferences or demands of the society. For women, oocyte and embryo cryopreservation is highly successful. Ovarian tissue cryopreservation and re-implantation, though successfully utilized, is still experimental. For men, sperm retrieved from ejaculate or surgical intervention of epididymis and testes have long been successfully cryopreserved. Outcome of fresh or cryopreserved sperm are similar. Indication of fertility preservation varies depending on medical or genetic condition, personal preference, career requirement or apprehensible risk of damage to gametes. This mini- review highlights multiple indications and available options for fertility preservation in non-cancer adult men and women.
Keywords:Fertility Preservation; Oocytes; Embryos; Ovarian Tissue; Testicular Tissue; Vitrification; Menopause; Infertility
Introduction
Human society has changed significantly due to industrial and information technology revolution. Lifestyle changes, personal preferences, society demands, increased cost of family raising, dietary patterns, increased use of insecticides and pesticides, increased use of electronics, career competition and ideological changes have affected human fertility and family structure. The environmental changes have negatively impacted the reproductive health, the effects of which extend beyond the individual and family to the society and even to the World [1,2]. These effects are more pronounced in women. Although men can often maintain fertility potential for lifetime, however, the evidence indicates worsening of semen parameters, including sperm genetics, and reduced reproductive success [3]. Improvements in oocyte, sperm, embryo, ovarian and testicular tissue cryopreservation in association with advancements in endocrinology and surgery have opened new avenues. Men with reproductive problems like disorders of sex development, cryptorchidism, hypospadias, low testosterone levels and poor semen quality [4] can preserve their fertility. There is a great need for fertility preservation counseling before initiation of any treatment that may affect the gametes production and quality. The objectives of this mini- review are to highlight indications and currently available fertility preservation options for adult noncancer individuals.
Fertility Preservation Indications for Women
The demand of fertility preservation by non-oncological adult women has increased tremendously in recent years. The indications are given below.
Delaying Childbearing: The women are increasingly delaying childbearing due to career competition, employment requirement, non-availability of suitable partner or other personal reasons. To avoid oocyte depletion, oocyte cryopreservation is an alternate to have biological children in future. In the United States, female obstetrics and gynecology residents are increasingly choosing to preserve their oocytes due to career and social support reasons [5]. A few prominent companies are offering to cover cost of oocyte cryopreservation to their female employees affecting the trend of fertility preservation. Though fertility preservation may allow a woman opportunity to have her biological children later during her life, however, sufficient information on the safety, efficiency, emotional risks and cost effectiveness is not available. Elective oocyte vitrification in mid-late thirties resulted in good outcome after these women utilized IVF in their early forties [6]. The minimum number of mature oocytes needed to achieve a reasonable outcome is estimated to be 8-10 in women aged about 35 years. More oocytes are needed as the age advances. Women choosing this option should cryopreserve their oocytes at younger age to increase possibility of success [7].
Delaying Menopause: A few days ago, in the United Kingdom, ovarian tissue cryopreservation, warming and re-implantation has been used to delay menopause. The idea is to remove a part of the human ovary, cryopreserve it and re-implant back in the same woman before menopause [8]. It is estimated that menopause can be delayed up to 20 years. The cryopreservation of part of the ovary is recommended for women below 40 years of age when their ovaries still have large number of follicles in the ovarian cortex.
Medical Conditions: The list of diseases is increasing in which either the disease or the treatment causes gonadal damage. The success of fertility preservation depends on age of patient, presence or absence of ovarian involvement, available time and the indication of fertility preservation. The most common medical conditions requiring fertility preservation are autoimmune diseases, hematopoietic stem cell transplantation and conditions causing premature ovarian failure [9]. The autoimmune diseases include inflammatory bowel disease, systemic lupus erythematosus, rheumatoid arthritis, pemphigus vulgaris, granulomatosis with polyangiitis, eosinophilic granulomatosis, Bechet’s disease or any other autoimmune disorder not responding to immunosuppressive treatment. The blood disorders include sickle cell anemia, plastic anemia and thalassemia major. The premature ovarian failure can be caused by disorders of hypothalamic-pituitary-gonadal axis, oophoritis, benign ovarian tumors, Turner syndrome, fragile X, beta thalassemia, endometriosis and galactosemia [10]. A mutation of germline transcription factor (GATA2) involved in hematopoiesis and angiogenesis was discovered in 2011. Due to the risk of ovarian failure, affected patients should be recommended fertility preservation [11].
Surgery of the Pelvic Area: Surgery in the pelvic area may damage the ovaries or may interfere with its normal physiological function [12]. The bilateral ovarian endometriosis surgeries have a low but definite risk of premature ovarian failure occurring immediately after surgery [13].
Other Conditions: The women with poor ovarian reserve and Turner syndrome can opt for oocyte cryopreservation. Multiple attempts may be needed to get enough number of mature oocytes to create good quality embryos. Depending on the age, 15-20 mature oocytes for cryopreservation are estimated to achieve a live birth [14].
Fertility Preservation Options for Women
The currently available fertility preservation options for women are preserving mature oocytes, embryos and ovarian tissue [15]. The vitrification is method of choice for oocytes and embryos cryopreservation. The programmable slow cryopreservation and vitrification produce similar pregnancy rates for ovarian tissue cryopreservation.
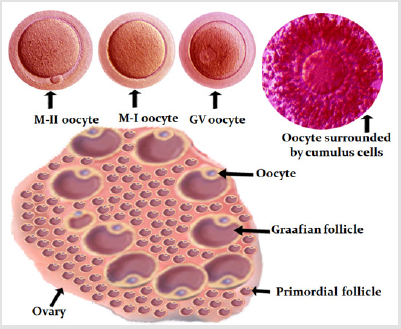
Figure 1: Diagrammatic presentation of human ovary (source of oocytes). In the top row are developmental stages of oocyte and an oocyte surrounded by cumulus cells. The oocyte at MII stage is desired for fertility cryopreservation.
Oocyte Cryopreservation: In vivo matured oocyte cryopreservation is the only choice for unmarried women. The married women by choice or due to non-availability of sperm on the day of ICSI can cryopreserve oocytes. In advanced age women with poor ovarian reserve, oocyte cryopreservation can be used to pool eggs either for routine IVF/ICSI or for preimplantation genetic testing. Figure 1 shows oocyte source and stages of development. Currently the mature oocytes (MII) survive well and result in live birth. The survival of immature oocytes (GV and MI) is not satisfactory and further studied are needed to improve their outcome [16]. Implantation and clinical pregnancy rates are similar after use of fresh or cryopreserved-warmed mature oocytes [17].
Ovarian tissue cryopreservation: Freezing ovarian tissue or
whole ovary are still experimental options. If the ovaries have to be
removed, either strips of the ovarian cortex or the whole ovary can
be frozen. The cryopreservation of ovarian cortical tissue presents
an efficient and high-yield method of potentially preserving
thousands of ovarian follicles through a single procedure. Live
births have been reported after re-implantation of ovarian strips
[18]. Pregnancy rates after re-implantation of ovarian tissue are
similar between slow freezing and fast freezing (vitrification)
methods; however, vitrified transplants have longer duration of
function [19] (Figure 2).
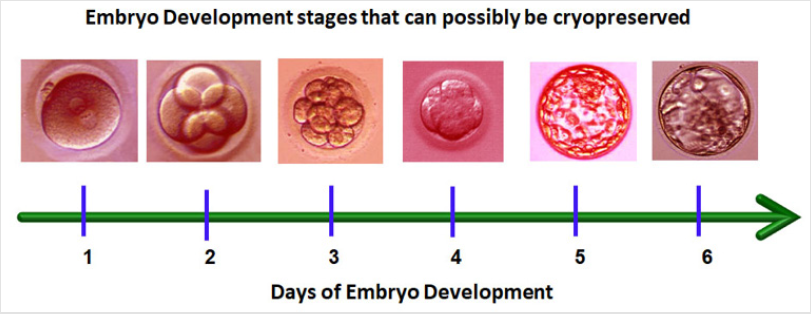
Figure 2: Different days and developmental stages at which the embryos can be preserved. After day-7 even if the embryo
becomes blastocyst, chances of establishing pregnancy are extremely low. Embryo Cryopreservation: Embryo cryopreservation is an
established method of fertility preservation for married women.
Pregnancy and live birth rate after frozen embryo transfer are
either equivalent or higher than those achieved after fresh embryo
transfer [20]. The embryos have been successfully frozen at different
stages of development (Figure 3). Both slow (programmable) and
fast (vitrification) procedures have been successfully used. Due to
higher survival rate and time saving, vitrification is now almost an
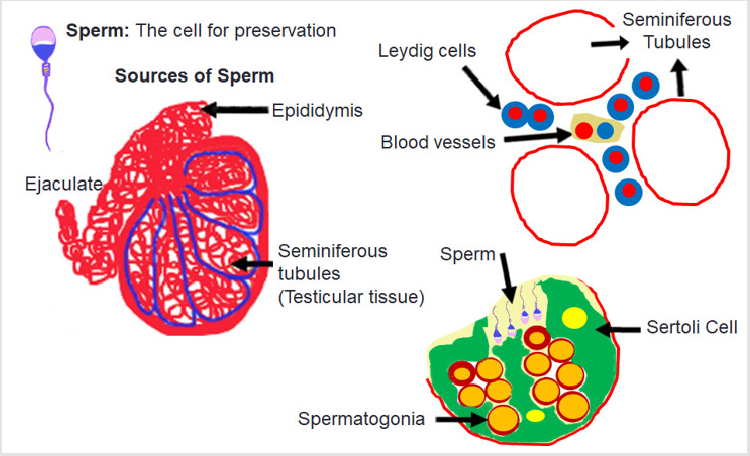
exclusive procedure for embryo cryopreservation. Figure 3: Diagrammatic presentation of human testis and seminiferous tubules. The sperm are made in the seminiferous tubules
containing Sertoli and Leydig cells necessary for spermatogenesis. Sperm cryopreservation is an established procedure for fertility
preservation. In men with poor semen quality and those who may
not be available at the time of assisted reproduction procedure of
their partner, semen cryopreservation is recommended. Surgical
sperm collection from testicle may not coincide with oocyte
aspiration day, or there may be minimal chances of finding sperm
by surgical retrieval, therefore, surgical sperm retrieval and
cryopreservation are recommended before initiation of ovarian
stimulation [21]. Medical Conditions: The medical conditions include systemic
lupus erythematosus, multiple sclerosis, diabetes mellitus and
cytotoxic therapy for non-cancer conditions like glomerulonephritis
[22]. Genetic Condition: The Klinefelter’s syndrome is a genetic
condition causing azoospermia in about 90% affected cases [21].
Some affected individuals produce very rare motile sperm. These
individuals can be offered to ejaculate on multiple occasions and
preserve if motile sperm are found. Alternatively, testicular tissue
can be obtained via TESE or Micro-TESE. Such sperm are usable by
intracytoplasmic sperm injection. Births have been reported from
individuals affected by Klinefelter’s syndrome [23]. Other Conditions: For men, who have not yet completed their
family and are at risk of Andropause, sperm cryopreservation at an
earlier age is a feasible option to safeguard fertility [24]. Testicular
tissue cryopreservation at an earlier age and re-implantation at
andropause has not been experimented yet. A few other conditions,
like severe testicular injury, extensive surgery in the pelvic area
and gender reassignment procedures may need testicular tissue
or ejaculated sperm cryopreservation. The gender reassignment
will make the individual temporarily or permanently infertile.
Therefore, in such procedures, enough sperm should be collected
via ejaculation or testicular tissue and preserved for future use
before initiating hormonal or surgical interventions [20]. Similarly,
injury to the spinal cord, surgery of the retroperitoneal lymph
node, aortic-iliac reconstruction and colorectal incisions may cause
anejaculation. Semen cryopreservation after ejaculation can be
repeated after 2-3 days [25]. Men suffering from hypothalamic-pituitary-gonadal axis
dysfunction are able to produce sperm at restoration of hormones
to physiological level [22]. Such individuals may lose sperm
production on discontinuation of therapy. Sperm cryopreservation
for such individuals is highly recommended. The most successful option for men is cryopreservation of
ejaculated sperm. Based on the quality of sample, it can later be
used for intrauterine insemination, in vitro egg insemination
or intracytoplasmic sperm injection. Multiple ejaculates can be combined to provide appropriate number of motile sperm for any
of the abovementioned procedure. The cryopreserved samples
have been successfully used after 40 years in storage [26]. Fertility preservation has become a need of adult women
and men in changing human society. Significant advances have
been made in fertility preservation technologies and results are
comparable after use of fresh or cryopreserved gametes. Use of
cryopreserved gametes has become an integral part of assisted
reproduction in the treatment of infertile couples. Fertility
preservation counseling before initiation of any therapy that may
damage gametes is essential. Fertility Preservation Indications for Men
Hypothalamic-Pituitary-Gonadal Axis Dysfunction
Fertility Preservation Options for Men
Conclusion
References

 Mini Review
Mini Review