Abstract
Objective: Using clay nanoparticles grafted with polyacrylic acid in the recent studies has shown significant improvement in bond strength. Incorporation of these materials into the experimental adhesive suggests the theory that adhesives containing active acidic groups deactivate the amine group and decrease the degree of conversion of adhesives with Camphorquinone (CQ) /amine photo initiator and resulting in higher microleakage.
Materials and Methods: 60 class V cavities were prepared on the buccal/lingual surfaces of extracted, sound premolar teeth and restored using an experimental dentin bonding agent and light-cure composite in three groups (n=20): Group1: 1wt% CQ/ amine, Group2: 1wt%butanedione and Group3: 0.5wt% CQ+1wt% butanedione. Microleakage was assessed at the occlusal and gingival margins of the restorations. degree of conversion of the adhesive was calculated using Fourier transform infrared spectroscopy. Data were analyzed using SPSS23 and Kruskal Wallis, Wilcoxon, Mann Whitney U and Fisher’s exact tests (α=0.05).
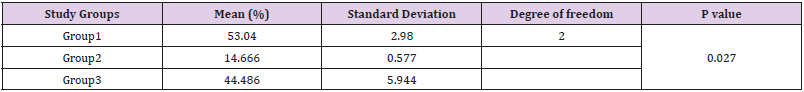
Results: Score of microleakage was not significantly different at the occlusal and gingival margins among the groups but it was significantly different at the occlusal margin compared to the gingival margin in all groups (P<0.001). Degree of coversion of groups 1 and 3 was significantly different from that of group 2; but groups 1 and 3 were not significantly different (P=0.027).
Conclusions: Using 1wt% butanedione instead of 1wt% CQ/amine in the understudy dentin adhesive system or the combination of both had no significant effect on microleakage of composite restorations. degree of conversion of dentin adhesive containing butanedione was significantly lower than other groups.
Keywords: Degree of Conversion; Dentin Bonding System; Microleakage; Photo Initiator
Abbreviations: CQ/amine: Camphorquinone /Amine, DC: Degree of Conversion, FTIR: Fourier transform infrared spectroscopy, BD: butanedione, PAA-g- nano clay: Poly Acrilic Acid Grafted with Nano clay
Introduction
Recent advances in dental adhesives and composite resins are responsible for the popularity of these materials for direct restoration of carious lesions [1]. At present, bond to dentin is weaker than the bond to enamel due to the heterogeneous and moist nature of dentin. Thus, a long-lasting, durable bond to dentin is less commonly achieved compared to enamel [2,3]. Of all the components involved in bonding at the resin-dentin interface, the adhesive layer has the lowest modulus of elasticity [4,5]. Thus, most adhesive systems available in the market contain fillers that increase the mechanical properties and the bond strength of the restorations [6-9].
Nanometer-sized fillers like clay are available; By performing adequate surface treatments, these fillers can be lodged in between polymer chains in dentin adhesive layer and increase the mechanical properties of the adhesive and subsequently enhance the bond strength [10,11].
Addition of clay nanoparticles to dentin bonding agents results in fast deposition of particles due to their higher density. However, this problem can be solved by surface treatment of nano clay particles with a polymer, which decreases density and results in a reaction between active groups bonded to the surface of particles and the solvent. One effective method to form active groups on the surface of nanoparticle is their grafting with monomers [4,5,12]. Studies on this topic have demonstrated that grafting nano-clay particles with poly acrylic, poly methacrylic and poly methyl methacrylic acids enhances the mechanical properties of adhesives [12-14].
Using clay nanoparticles grafted with polyacrylic acid in the recent studies has shown significant improvement in bond strength [14]. Incorporation of these materials into the experimental dentin bonding agent suggests the theory that adhesives containing active acidic groups deactivate the amine group and decrease the degree of conversion of adhesives with CQ/amine photo initiator resulting in a weak bond between dentin and composite and allowing higher diffusion of water at the composite/dentin interface and subsequently higher microleakage. To overcome this problem, a single step photo initiator like 2,3 butanedione can be used.
In the recent studies, several types of new photo initiators such as 2,3 butanedione, 1-phenyl-1,2-propanedione and hexafluoroantimonate p-octyloxy-phenyl iodonium have been evaluated [15,16]. CQ is the most commonly used photo initiator in light-cure composite resins [15]. CQ is naturally yellow causing problems in color matching. This limits the application of its higher concentrations and consequently, the DC and curing depth [15]. The 2,3 butanedione (Diketone BD) is a viscous liquid. Due to its physical state, it is well compatible with resin components and in contrast to CQ, which is solid at room temperature, it can be used as a diluting agent in composite resins. The 2,3 butanedione is a photo initiator without the amine activator. By light irradiation and cleavage of C-C bond, it produces two carbonyl free radicals and initiates polymerization [15,17]. In the UV –VIS spectrum, butanedione has a peak absorbance at 419 nm, which is close to the output power of dental light curing units. On the other hand, shorter absorbance wavelength of butanedione lowers its yellowness compared to that of CQ [15]. The aim of the present study was to evaluate the effect of type of photo-initiator (2,3 butanedione, CQ/amine or both) in an experimental dentin bonding agent on the DC of adhesive and microleakage of class V composite restorations bonded with this experimental dentin bonding agent.
Materials and Methods
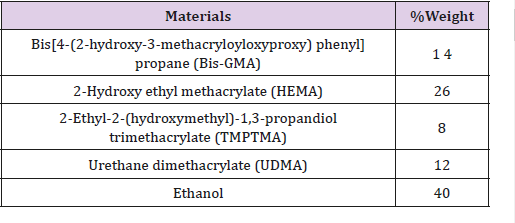
The graft polymerization of acrylic acid onto the surface of nanoclay was carried out according to the previous works [14]. The grafted nanoclay powder was added to the adhesive solution and the obtained mixture was sonicated for 3 minutes under 50% power and 6 pulses (Each pulse for 1 second). Dentin adhesive system was manufactured in the Iranian Polymer Research Center according to the formulation presented in Table 1. Polyacrylic acidgrafted nano clay filler particles were added by 0.2wt%. Photo initiator was added as follows:
Table 1: The composition of the experimental bonding agent.
Formulation 1: 1wt% CQ/amine
Formulation 2: 1wt% butanedione
Formulation 3: 1wt% butanedione+0.5wt%CQ
Preparation of Teeth
30 Sound human premolar teeth were collected and stored in 10% formalin. Their buccal and lingual surfaces were examined under a stereomicroscope for presence of any caries, cracks, abrasion, fracture, or developmental anomalies. Using a cylindrical diamond bur measuring 1mm in diameter (Diatech, Scissortail, Switzerland) along with water and air spray, standard class V cavities measuring 2×3×3 mm were prepared on the buccal and lingual surfaces of teeth; Enamel at the occlusal margin was beveled (45°, 1mm). Each class V cavity was considered as one specimen and the bur was discarded after preparation of 5 cavities. The cavities were etched using 35% phosphoric acid gel (3M ESPE. St Paul, MN.USA) for 15 seconds and were then rinsed with water for 10 second and dried leaving the dentin surface relatively moist. Prepared specimens were randomly divided into three groups (n= 20):
1: The experimental dentin bonding agent with 1wt% CQ/ amine,
2: The experimental dentin bonding agent with 1wt% butanedione and
3: The experimental dentin bonding agent with 1wt% butanedione+0.5wt%CQ
The adhesive in each group was applied to the cavity surfaces using a micro brush in two thin coats and after 30 seconds, the adhesive solvent was evaporated with gentle air spray for 15 seconds. Light curing was performed using Spectrum 800 halogen light-curing unit (Dentsply/Caulk, USA) for 20 seconds. The cavities were filled using a microhybrid light cure composite (Filtek Z250, 3M ESPE, St. Paul, USA) in 1-mm increments. Each increment was light cured for 20 seconds and finishing and polishing were performed using Soflex burs (Passanti 332, Kerr, Italy-Switzerland).
Measurement of Microleakage
Specimens were subjected to 3000 thermal cycles using digital thermocycler (Willytec version 3.0, Willytec GmbH, Gräfelfing, Germany) at 5-55°C. After thermocycling, tooth apices were sealed with sticky wax and the surfaces of the teeth were coated with two layers of nail varnish except for one-millimeter margin around the cavities. Specimens were immersed in 10% methylene blue for 72 hours and were mounted in self-curing acrylic resin (Acropars, Kaveh, Tehran, Iran) for future cutting. Using a diamond disc, specimens were buccolingually sectioned at the middle of the restoration using a cutting machine (Nonstop, Albany, NY, USA).
Microleakage of each specimen was measured using a stereomicroscope (Leica Zoom 2000, Wetzlar, Germany) at 20X magnification separately by two observers in a double-blind fashion. The score of microleakage at the occlusal and gingival margins was scored using the following scoring system [18]:
0: No microleakage
1: Dye penetration to 1/3 of cavity depth
2: Dye penetration to 2/3 of cavity depth
3: Dye penetration to the entire depth of cavity
4: Dye penetration exceeding the depth of cavity towards the pulp
The mean score of microleakage at both the occlusal and gingival margins was considered as the microleakage score for the respective specimen.
Measurement of the DC of the Three Formulations of the Experimental Adhesive
One drop of each formulation of the experimental adhesive was placed on a thin polyethylene sheet. The solvent was gently vaporized using mild air spray for 15 seconds. To obtain a very thin coat, a second sheet was placed over it and the complex was transferred to a FTIR spectrophotometer (Spectrum GX, Perkin Elmer). The absorbance of the sample before and after light curing was measured using the transmission mode of FTIR. DC was calculated based on the ratio of the absorption intensity of C=C aliphatic group (absorption peak height at 1638cm-1) divided by the internal reference of C=C aromatic group (absorption peak height at 1608cm-1) before and after curing of the specimen [18].
DC (%) = (1−) x100
Data of the score of microleakage and DC were entered inSPSS software and the mean and standard deviation values were calculated. Kruskal Wallis test was used to compare the degree of microleakage between groups. Using Fisher’s exact test, occlusal microleakage was compared between groups. For intragroup comparison of microleakage between the occlusal and gingival margins, Wilcoxon test was applied. To compare DC between groups, Kruskal Wallis and Mann Whitney U tests were used. Significance level was set at 0.05.
Results
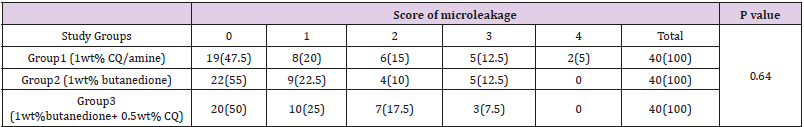
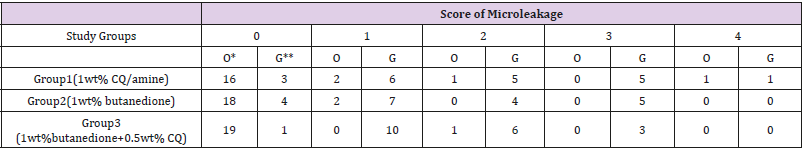
Data for scoring of microleakage in the study groups is reported in Table 2. The results of Kruskal Wallis test for comparison of microleakage between groups revealed no significant difference (P=0.64). Table 3 shows the score of microleakage in the study groups at the occlusal and gingival margin of restorations. Fisher’s exact test compared the microleakage at the occlusal margin and found no significant differences between groups (P=0.52), the Kruskal Wallis test compared the microleakage at the gingival margin and found no significant difference in this respect among groups (P=0.76). Comparison of microleakage at the gingival and occlusal margins (Figure 1) in each study group revealed a significant difference (P<0.05). The mean Degree of conversion for the first group (1wt% CQ/amine) was 53.04±2.98 %, and 14.66±0.57 % in the second group (1wt% butanedione), and 44.48±5.94 % in the third study group (1wt% butanedione+0.5wt%CQ). Based on the results of the Kruskal Wallis test, DC was significantly different between the study groups (Table 4). Pairwise comparison of the study groups for degree of conversion, by Mann Whitney U test indicated a significant difference between groups 1 and 2 (p=0.046), and between groups 2 and 3 (p=0.046). However, the difference between groups 1 and 3 was not significant (p=0.055).
Table 2: Frequency Distribution of the Degree of Total Microleakage in the Studied Groupsa,b.
Note: aValues are expressed as No. (%).
bKruskal–Wallis test.
Discussion
Microleakage is a factor responsible for pulpal reactions to restorative materials. Formation of microscopic gaps at the toothrestoration interface enables the passage of ions, molecules, bacteria and their toxic products as well as liquids, causing postoperative tooth hypersensitivity, marginal discoloration, secondary caries and pulp inflammation [19-21]. Degree of conversion (DC) is an important criterion for clinical success of a composite resin restoration. Clinically, polymerization of materials used in esthetic dentistry must be increased as much as possible to achieve the maximum restoration strength. Although increased DC increases the polymerization shrinkage of adhesive or restoration and subsequently imparts tensile stresses to the bonded interface, it can be compensated by application of suitable clinical technique; while inadequate polymerization causes problems such as less biocompatibility, lower physical and mechanical properties and early failure of the restoration [22].
In the current study, a filler-containing dentin adhesive was used. Studies have reported that filler-containing adhesives have improved mechanical properties and enhance the bond strength [6-9,13,14]. Kim et al. [23] reported that addition of less than 1wt% nanofillers to dental adhesives increased the microtensile bond strength of the composite restoration; but in higher amounts of nanofillers, the microtensile bond strength decreased due to the aggregation of particles and formation of clusters. Solhi et al. [14] demonstrated that dental adhesives containing 0.2 wt% PAA-gnano clay particles, showed a significantly increased bond strength. The photo initiator is often added in the range of 0.1-1% [22]. In the present study, we applied 1-1.5wt% of photo initiator within the formulation of the experimental adhesive.
There are some relevant methods to determine the degree of monomer to polymer conversion of composite resin specimens like FTIR spectroscopy, Laser Raman spectroscopy, Electron Spin Resonance (ESR), Infrared Spectroscopy (IR), Dynamic Mechanical Thermal Analysis (DMTA), Attenuated Total Reflection (ATR) etc [24]. In the current study, FTIR was used because it is a beneficial method for molecular detection and tracing of chemical reactions. It provides a better interpretation of the changes that occur in the polymer matrix. FTIR can easily detect and quantify chemical bonds [25]. This method has been used by Solhi et al. [14], Park et al. [26], Yazdi et al. [27] and Shin et al. [16] to assess the polymerization efficacy of Bis-GMA-based composite resins. Dye penetration is the most commonly used technique for assessment of microleakage. This method has acceptable techniqual sensitivity and is easier than other methods available for this purpose [28]. Thus, researchers use fuchsin or methylene blue with particles equal in size or smaller than the size of bacteria (about 2 microns) [19]; In the current study, 10% methylene blue was used. The only drawback of this method is its grading system because its sensitivity decreases by re-evaluation after a time interval [19]. In-vitro studies on microleakage are often performed on class V restorations. Specimens are usually subjected to thermocycling or mechanical loading to simulate the oral environment [28]; In the current study, specimens were subjected to 3000 thermal cycles prior to microleakage assessment. The results showed that in all groups some degrees of microleakage existed and none of the formulations evaluated in this study could provide complete marginal seal. No significant difference was seen among the study groups in terms of microleakage.
Fortin et al. [29] compared microleakage in several dental adhesive systems and reported that some degrees of microleakage were detected in all groups; which is in accord with the current study results. According to a study by Mousavinasab et al. [18] score of microleakage was not significantly different in several experimental filler-containing adhesives and Single Bond. This finding is in line with our results. Our study also showed that in all groups, microleakage at the gingival margin (Figures 2-5) was significantly higher than that in the occlusal margin; This indicates that marginal seal in the dentin margin was lower than that in the enamel margins. Lower microleakage in the enamel compared to dentin margin is due to the stronger bond to enamel because the enamel has a higher mineral content and is more uniformly mineralized compared to dentin. Also, dentinal fluid does not exist in enamel and thus, monomers can more easily penetrate its microporosities created following etching, resulting in a more reliable micromechanical bond. Another reason for the lower microleakage in the enamel margin is beveling the enamel margin increases the bondable enamel surface [20].
Also, by exposure of enamel crystals, a stronger bond is achieved. Manhart et al. [30] demonstrated that marginal microleakage in class V restorations bonded with several dentin bonding systems was significantly different at the occlusal and gingival margins; which also confirms our results. In the current study, DC was significantly different among the three groups and pair wise comparisons revealed significant difference of groups 1 and 3 with group 2 in this regard. However, groups 1 and 3 were not significantly different. Combining 1wt% 2, 3 butanedione and 0.5wt% CQ increased the DC compared to the use of 2,3 butanedione alone; It appears that using this combination as photo initiator (group 3) in the understudy experimental dentin bonding agent can be helpful because this formulation lacks linear amine, which probably becomes inactivated in presence of acid. Thus, in combination of CQ and 2, 3 butanedione, the reaction for generation of free radicals with butanedione is usually done with CQ even when amine is not present in the formulation.
In the current study, incorporation of a combination of butanedione and CQ showed more favorable results. Thus, future studies are suggested to assess the properties of higher concentrations of butanedione in combination with CQ. Moreover, since the effect of duration of solvent vaporization was not investigated in the current study, further studies are required to assess the effect of this factor.
Conclusion
Under limitations of the present study, Substitution of 1wt% butanedione for 1wt% CQ/amine in the studied experimental adhesive system or the combination of butanedione and CQ had no effect on the microleakage of class V composite restorations. Also, DC of adhesive containing 1% butanedione was significantly lower than that of the adhesive containing 1% CQ or combination of both.
Acknowledgement
We appreciate the dental research center of the Hamadan University of Medical Sciences and the Iranian polymer research center.
Conflict of Interest
The study was funded by Vice chancellor for Research and Technology, Hamadan University of Medical Sciences.
References
- Baracco B, Perdigão J, Cabrera E, Giráldez I, Ceballos L (2012) Clinical evaluation of a low-shrinkage composite in posterior restorations: one-year results. Operative Dent J 37: 117-229.
- Krifka S, Börzsönyi A, Koch A, Hiller KA, Schmalz G, et al. (2008) Bond strength of adhesive systems to dentin and enamel human vs. bovine primary teeth in vitro. Dent Mater J 24(7): 888-894.
- Poitevin A, De Munck J, Van Landuyt K, Coutinho E, Peumans M, et al. (2008) Critical analysis of the influence of different parameters on the microtensile bond strength of adhesives to dentin. Adhes DentJ 10(1): 7-16.
- Dickens SH, Cho BH (2005) Interpretation of bond failure through conversion and residual solvent measurements and Weibull analyses of flexural and microtensile bond strengths of bonding agents. Dent Mater J 21: 354-364.
- Schmidt G, Malwitz MM (2003) Properties of polymer–nanoparticle composites. Current opinion in colloid & interface science J 8: 103-108.
- Li Y, Swartz ML, Phillips RW, Moore BK, Roberts TA (1985) Materials science effect of filler content and size on properties of composites. J Dent Res 64(12): 1396-1403.
- Miyazaki M, Ando S, Hinoura K, Onose H, Moore BK (1995) Influence of filler addition to bonding agents on shear bond strength to bovine dentine. Dent Mater J 11(4): 234 -238.
- Nunes MF, Swift EJ, Perdigão J (2001) Effects of adhesive composition on microtensile bond strength to human dentin. Am J dent 14(6): 340-343.
- Montes MA, de Goes MF, da Cunha MR, Soares AB (2001) A morphological and tensile bond strength evaluation of an unfilled adhesive with low-viscosity composites and a filled adhesive in one and two coats. J Dent 29(6): 435-441.
- Ultracki LA (2004) Clay-containing polymeric nano composites (1st) National Research Council. Canada, industrial materials institute, p. 73.
- Raghavan D, Feresenbet E, Yebassa D, Emekalam A, Holmes G (2001) Dispersion of Functionalized Nanoclay Platelets in an Amine-Cured Epoxy Resin System. MRS Online Proceedings Library Archive pp. 703.
- Atai M, Solhi L, Nodehi A, Mirabedini SM, Kasraei S, et al. (2009) PMMA-grafted nanoclay as novel filler for dental adhesives. Dent Mater J 25(3): 339-347.
- Solhi L, Atai M, Nodehi A, Imani M (2012) A novel dentin bonding system containing poly (methacrylic acid) grafted nanoclay: Synthesis, characterization and properties. Dent Mater 28(10):1041-1050.
- Solhi L, Atai M, Nodehi A, Imani M, Ghaemi A, et al. (2012) Poly (acrylic acid) grafted montmorillonite as novel fillers for dental adhesives: synthesis, characterization and properties of the adhesive. Dent Mater J 28(4): 369–377.
- Sun GJ, Chae KH (2000) Properties of 2, 3-butanedione and 1-phenyl-1, 2-propanedione as new photosensitizers for visible light cured dental resin composites. Polymer 41(16): 6205-6212.
- Shin DH, Rawls HR (2009) Degree of conversion and color stability of the light curing resin with new photoinitiator systems. Dent mater 25(8): 1030-1038.
- Fadaie P, Atai M, Imani M, Karkhaneh A, Ghasaban S (2013) Cyanoacrylate–POSS nanocomposites: Novel adhesives with improved properties for dental applications. Dent Mater 29: 61-69.
- Mousavinasab SM, Atai M, Alavi B (2011) To compare the microleakage among experimental adhesives containing nanoclay fillers after the storages of 24 hours and 6 months. J The Open Dent 29 (5): 52-57.
- Yavuz I, Aydin AH (2005) New Method for Measurement of Surface Areas of Microleakage at the primary teeth by biomolecule characteristics of methilene blue. Biotechnology & Biotechnological Equipment 19(1): 181-187.
- Heymann HO, Swift EJ Jr. Art and science of operative dentistry. 2013, 6th Ed. Elsevier Canada. Chap 4.
- Zavattini A, Mancini M, Higginson J, Foschi F, Pasquantonio G, et al. (2018) Micro-computed tomography evaluation of microleakage of Class II composite restorations: An in vitro study. European journal of dentistry 12(3): 369-374.
- Kirsten Lt, Van Landuyt KL, Snauwaert J, De Munck J, Peumans M, et al. (2007) Systematic review of the chemical composition of contemporary dental adhesive. J Biomater 28(2): 3757-3785.
- Kim JS, Cho BH, Lee IB, Um CM, Lim BS, Oh MH, et al. (2005) Effect of the hydrophilic nanofiller loading on the mechanical properties and the microtensile bond strength of an ethanol-based one-bottle dentin adhesive. J Biomed Mater Res B Appl Biomater 72(2): 284-291.
- Yokesh C A., Hemalatha P, Muthalagu M, Justin MR (2017) Comparative evaluation of the depth of cure and degree of conversion of two bulk fill flowable composites. J Clin Diag Res: JCDR 11(8): ZC86.
- Paluszkiewicz C, Stodolak E, Hasik M, Blazewicz M (2011) FT-IR study of montmorillonite-chitosan nanocomposite materials. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy 79(4): 784-788.
- Park YJ, CHae KH, Rawls HR (1999) Development of a new photoinitiation system for dental light-cure composite resins. Dent Mater J 15(2): 120-127.
- Yazdi FM, Moosavi H, Atai M, Zeynali M (2015) Dentin bond strength and degree of conversion evaluation of experimental self-etch adhesive systems. J Clin Exp Dent 7(2): e243.
- Arslan S, Demirbuga S, Ustun Y, Dincer AN, Canakci BC, et al. (2013) The effect of a new-generation flowable composite resin on microleakage in Class V composite restorations as an intermediate layer. J Conserv Dent 16(3): 189-193.
- Fortin D, Swift EJ, Denehy GE, Reinhardt JW (1994) Bond strength and microleakage of current dentin adhesives. Dent Mater J 10(4): 253-258.
- Manhart J, Chen HY, Mehl A, Weber K, Hickel R (2001) Marginal quality and microleakage of adhesive classⅤ J Dent 29(2): 123-130.

 Research Article
Research Article