Abstract
Objective: The purpose of this study was to evaluate the aerosol inhalation of Tanreqing injection combined with conventional western medicine in the treatment of AECOPD by meta-analysis.
Methods: Electronic databases including Cochrane Library, EMBASE, Web of Science, Medline, Wanfang database, Chinese Biomedical Literature (CBM), Chinese National Knowledge Infrastructure (CNKI) and Weipu Database for Chinese Technical Periodicals (VIP) were searched, until August 2018. The randomized controlled clinical trials were selected based on specific criteria, in which aerosol inhalation of TRQI plus conventional western medicine treatment group was compared with a conventional western medicine treatment control group for patients with AECOPD. RevMan 5.3 software was used for data analysis and Cochrane handbook 5.2 was applied to assess the quality of selected trials.
Results: Based on the search strategy, 12 trials containing 1020 patients were included. The results proved that compared with conventional western medicine therapy alone, aerosol inhalation of TRQI plus conventional therapy improved PaO2, clinical efficacy and lung function and reduced PaCO2, shortened the length of hospital stay, the time to resolution of fever, cough, crackles and was thus more therapeutically beneficial. Three trials described side effects of TRQI, while no severe side effects reported.
Conclusions: Inhalational administration of TRQI intervention appears to provide more benefits for AECOPD patients than conventional western medicine treatment alone, although more high-quality trials are needed to prove this result.
Keywords: Tanreqing injection; Chronic obstructive pulmonary disease; systematic review; Traditional Chinese medicine; Aerosol inhalation
Abbreviations: COPD: Chronic Obstructive Pulmonary Disease; AECOPD: Acute Exacerbations of Chronic Obstructive Pulmonary Disease; TRQI: Tanreqing Injection; CHM: Chinese Herbal Medicine; SGRQ: St George’s Respiratory Questionnaire; 6MWT:Scoring and 6 Min Walk Test; TCM :Traditional Chinese Medicine; RCTs: Randomized Controlled Trials; GOLD: Global Initiative for Chronic Obstructive Lung Disease; FVC: Forced Vital Capacity; FEV1: Forced Expiratory Volume in 1s; RR: Relative Risk; MD: Mean Difference; CI: Confidence Interval; PaO2: Arterial Partial Pressure Of Oxygen; PaCO2: Arterial Partial Pressure Of Carbon Dioxide; NS: Normal Saline.
Introduction
Chronic obstructive pulmonary disease (COPD) is a chronic, irreversible lung disease which is characterized by progressive deterioration of lung function [1]. Morbidity and mortality associated with COPD is increasing and it is the fourth leading cause of death worldwide [2]. Besides chronic impairment, this disease can cause acute exacerbations, defined as acute events characterized by the worsening of the respiratory symptoms beyond daily variations, which leads to a change in medication or even hospitalization [1]. Exacerbation is a main contributor to deterioration of airflow limitation, increase in health care costs, worsening of quality of life, need for hospitalization and risk of death [3-5]. Current treatment for AECOPD includes controlled oxygen administration, inhaled bronchodilators, intravenous aminophylline, systemic corticosteroids and mechanical ventilation and antibiotics if necessary. Despite the effectiveness of these therapies, acute exacerbation still occurs frequently. Moreover, these therapies have been related with some adverse drug reactions such as tremor, heart palpitations, candidiasis and antibiotic resistance [6]. Clinicians are hard to balance the safety and effectiveness of these pharmaceutical therapies for AECOPD patients. There is a long history of using traditional Chinese medicine (TCM) to treat disorders now classified as COPD, particularly in China, India, and their neighboring countries [7,8]. In western countries, TCM has been gradually accepted as a form of complementary medicine [9]. Chinese herb formulas combined with routine pharmacotherapy have showed more benefits on respiratory symptoms, 6 min walk test (6MWT), lung function, and arterial blood gases when compared with routine pharmacotherapy alone [10,11]. Tanreqing injection (TRQI) is a standardized formulation consisting of Flos lonicerae, Cornu saigae tataricae, Radix scutellariae, Fructus forsythiae and Fel ursi [12]. Previous studies have proved that TRQI can be prescribed to clear phlegm-heat symptoms for the COPD patients and also shows the effects on anti-inflammation, antioxidative stress and improve airway mucus hypersecretion which may shorten the length of hospital stay and reduce recurrence of acute exacerbation [13-15]. Thus, TRQI has been widely used in China for the treatment of pulmonary diseases, especially for COPD.
The inhalational administration has gained increasing interest in recent years in the treatment of pulmonary disease such as asthma, COPD and respiratory tract infections in clinic [16,17]. Traditional Chinese medicine delivery to the lung is in the stage of development. Many randomized controlled trials of inhalational administration of Traditional Chinese medicine in the treatment of AECOPD have been conducted to investigate its safety and efficacy. The aim of the current systematic review was to assess the safety and efficacy of aerosol inhalation of TRQI in the treatment of AECOPD on the comparison of two groups: TRQI administered via aerosol inhalation plus conventional western medicine (therapy A) versus conventional western medicine alone (therapy B). It was anticipated that this systematic review could provide evidence based on information for clinical practice [15].
Methods
Eligibility Criteria
Type of Study: We excluded quasi-randomized and nonrandomized controlled trials and included all the randomized controlled trials (RCTs) that compared therapy A with therapy B in the treatment of AECOPD.
Participants: COPD patients must be confirmed according to the standard diagnostic criteria including the American Thoracic Society, Chinese COPD guideline [18] or the Global Initiative for Chronic Obstructive Lung Disease (GOLD) [1]. All patients must be older than 18 years and diagnosed as AECOPD with one or more following symptoms: increased sputum purulence or dyspnea or chest tightness or fluid retention [1]. Studies of patients with bronchiectasis, pulmonary tuberculosis, asthma or any other pulmonary diseases were excluded.
Interventions: Aerosol inhalation of TRQI combined with conventional western medicine was compared with conventional western medicine alone. Western medicine treatments were standardized as far as possible for both arms.
Outcome: The main outcomes included
a) Lung function—forced expiratory volume in 1s as a percentage of the predicted value (FEV1% predicted) and FEV1/FVC (forced vital capacity)
b) Arterial blood gases—arterial partial pressure of oxygen (PaO2) and carbon dioxide (PaCO2)
c) Length of hospital stays.
The secondary outcomes include:
a) Marked efficacy rate [19]
b) Time to resolution of symptoms such as fever, cough, crackles and
c) Adverse events.
Search Strategy
Online databases including Medline, EMBASE, Web of Science, Cochrane Library, Chinese National Knowledge Infrastructure (CNKI), Wanfang database, Weipu Database for Chinese Technical Periodicals (VIP) and Chinese Biomedical Literature (CBM) from their inceptions to August 2018 were selected and searched for identification of eligible studies by LZB and MYY. In addition, we searched some Traditional Chinese Medicine (TCM) journals not indexed in the electronic databases. Studies aiming to compare the clinical efficacy of aerosol inhalation of TRQI combined with conventional western medicine versus conventional western medicine alone were regarded as potential sources of this systematic analysis and included after carefully review. The languages of included studies were limited to English and Chinese, with the restriction of human trials. The search strategies for English and Chinese Databases are provided as examples (see Additional file 1).
Study Selection and Data Extraction
Two independent reviewers (YLS and LZB) assessed the title and abstract of the literature after removing duplications. The further screening was performed to select eligible articles by reviewing the full text. Any disagreement between the reviewers was resolved by discussion with a third person (CC). We designed the standardized database sheet for data extraction. The following details of the study design were extracted from each study: first author, publication year, trial duration and sites, sample size, participants, details of intervention, control and outcomes, and follow-up period and adverse events. Any lack of information was supplemented by correspondence with the original principal investigators. All review authors participated in resolving discrepancies until a consensus was reached.
Assessment of Risk of Bias About the Included Studies
Two reviewers (QGR and LZB) independently assessed the risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Version 5.2). Random sequence generation (selection bias), allocation concealment (selection bias), blinding of participants and personnel (performance bias), blinding of outcome assessment (detection bias), incomplete outcome data (attrition bias), selective reporting (reporting bias) and other bias were considered for assessment. Each domain was judged to be of low risk, high risk or uncertain risk (“Yes” for a low risk of bias, “No” for a high risk of bias, “Unclear” otherwise). Based on these criteria, each study could be divided into three grades as follows: low risk of bias (low risk of bias for all key domains), unclear risk of bias (unclear risk of bias for one or more key domains), and high risk of bias (high risk of bias for one or more key domains). Discrepancies were resolved by discussion between reviewers.
Data Synthesis
All calculations were conducted using Review Manager software (Version 5.3) provided by the Cochrane Collaboration. Dichotomous variable was presented as relative risk (RR). Continuous outcome was presented as mean difference (MD) and its 95% Confidence Interval (CI). Statistical heterogeneity was assessed according to the Cochrane Handbook of Systematic Review of Interventions (Version 5.2). Statistical heterogeneity was evaluated with I-squared (I2) statistic. If there was no significant heterogeneity among the included trials (I2 ≤ 50%), fixed effects model was used, if not (I2> 50%), random effects model was used. Subgroup analysis was conducted according to the different types of interventions used in the trial. Publication bias would be assessed by funnel plot, if the number of studies were sufficient.
Subgroup Analysis
We had performed the subgroup analysis on two different doses of TRQI in this systematic review. Patients with therapy A were divided into two groups (aerosol inhalation of TRQI 10ml qd; aerosol inhalation of TRQI 10ml bid).
Results
Description of Included Studies
We identified 286 potentially relevant articles from eight databases. After removal of duplicates, 74 records remained. After going through the titles and abstracts, we excluded 18 papers with the following reasons:
a) Reviews, editorials and meta-analysis
b) Non-clinical trials
c) No control groups.
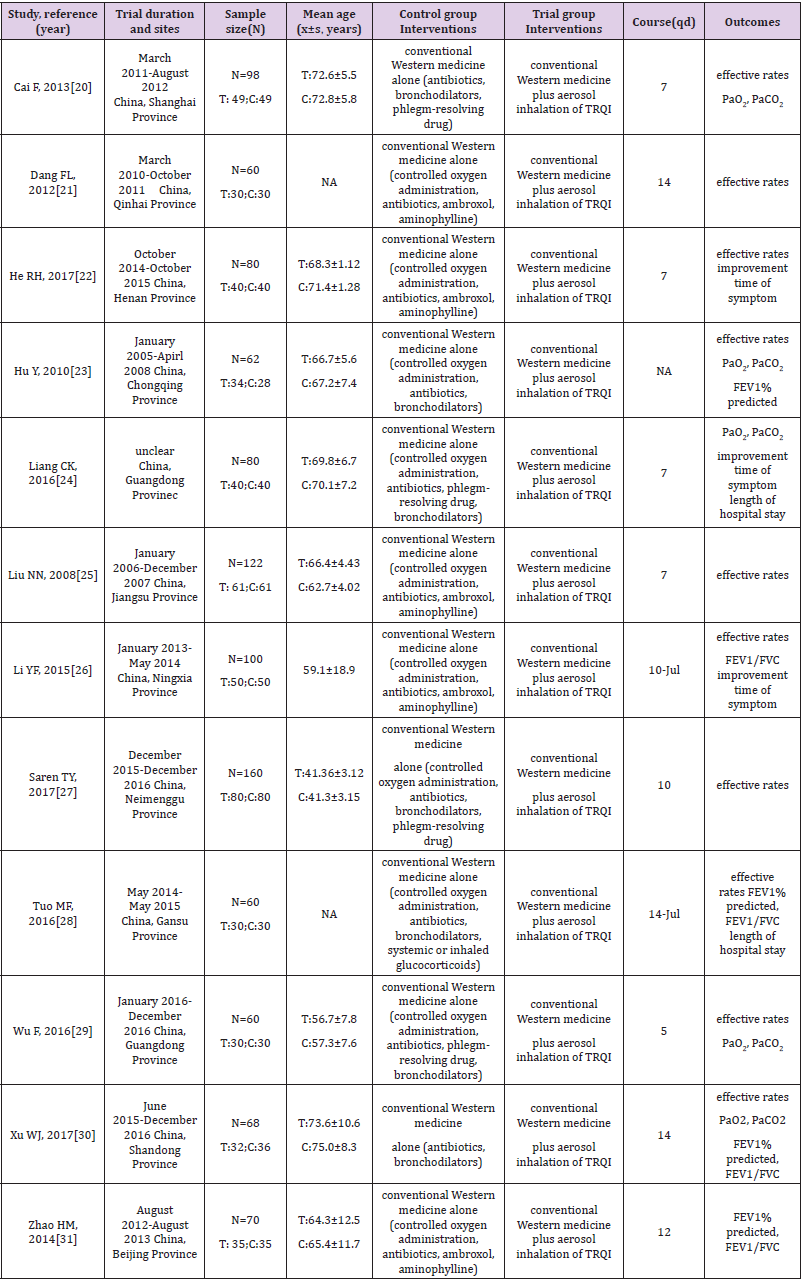
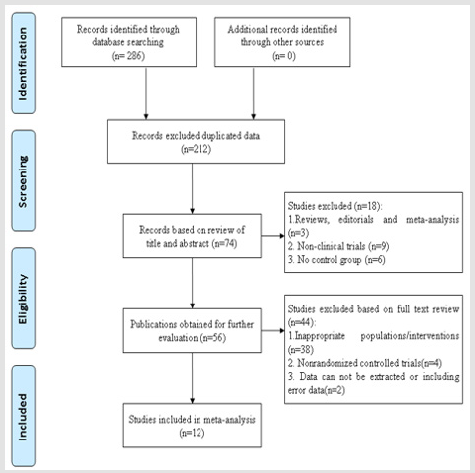
By reading the full text of the remaining 56 articles as possibly reporting the efficacy and safety of aerosol inhalation of TRQI combined with conventional western medicine in the treatment of acute exacerbations of COPD, 38 studies were excluded due to inappropriate populations/interventions; 4 studies were excluded because of nonrandomized controlled trials; 2 were excluded because of intelligible data extraction or including error data. Ultimately, 12 eligible studies were identified [20-31]. All the trials were published in Chinese. The characteristics of the included trials are listed in Table 1. The selecting process for included studies was presented as following (Figure 1).
Methodological Quality
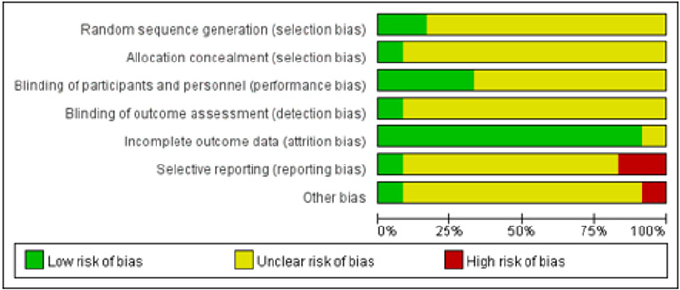
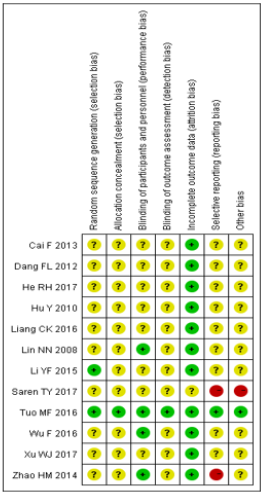
The methodological quality of included studies was assessed by the criteria in the “Cochrane Handbook for Systematic Review of interventions” (see Figures 2 & 3). All except two trials [26,28] did not describe how the random allocation sequence was generated, only marked “randomized” without exact methods, and only one study 28 mentioned the methods of allocation concealment, which implied that the selection bias might have been produced. Moreover, only four [25,28,29,31] of the 12 studies described the blinding (patients, clinicians or outcome assessors), which suggested that performance bias and measurement bias might exist. Finally, only one trial [27] had addressed incomplete outcome data. Selective reporting in other studies was unclear due to the unavailability of the research protocol. The sample size calculation was not reported by any of the included studies.
Effects of Interventions
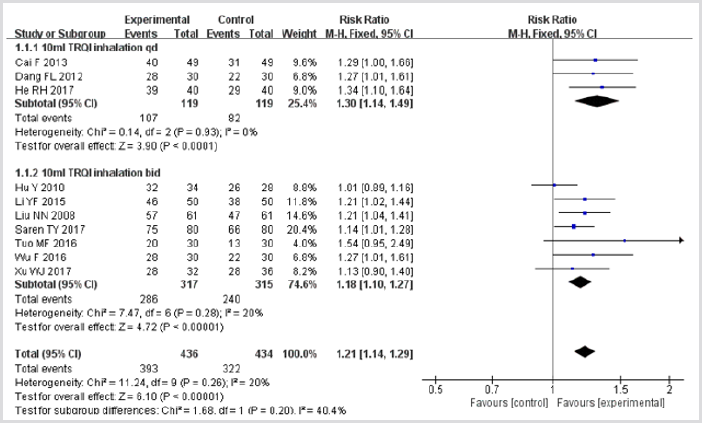
Figure 4: Meta-analysis of outcome (clinical effective rates) in randomized controlled trials comparing therapy A with therapy B in the treatment of acute exacerbations of COPD.
Clinical Effective Rates: Ten trials 20-23,25-30 involving 870 patients provided data for this outcome. In the therapy A group, 393 of 436 (90.1%) patients reached clinical effectiveness, while 322 of 434 (74.2%) subjects achieved clinical effectiveness in therapy B group. The value of I2 was less than 50%, indicated that the heterogeneity across different trials was not significant (P>0.05), and the fixed effect model was applied in this pooled analysis. As shown in Figure 4, the result of meta-analysis demonstrated a favorable clinical effectiveness for TRQI treatment (RR = 1.21, 95%CI: 1.14–1.29; p<0.05). In the subgroup analysis, there were also significant differences with regards to both TRQI 10ml qd aerosol inhalation and TRQI 10ml bid aerosol inhalation between combination group and control group. The pooled RR for TRQI 10ml qd aerosol inhalation was 1.30 with 95%CI ranged from 1.14 to 1.49 (p<0.05), and the integrated RR for TRQI 10ml bid aerosol inhalation was 1.18 (95%CI: 1.10–1.27; p<0.05). These findings indicated that conventional western medicine plus aerosol inhalation of TRQI had better RR than conventional western medicine alone.
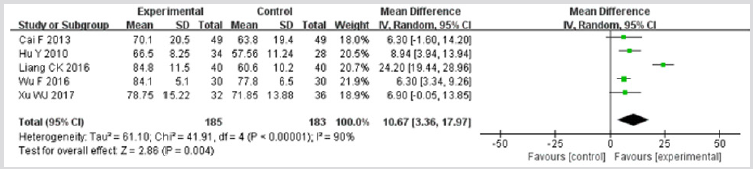
PaO2: Five studies [20,23,24,29,30] included in this systematic analysis provided the data on change of PaO2 after treatment. As there was statistical heterogeneity (I2 = 90%, p<0.01), the random effect model was used in this meta-analysis. The results showed that the MD was 10.67 (95%CI: 3.36–17.97; p=0.004), indicating that application of TRQI could increase the level of PaO2 (Figure 5).
Figure 5: Meta-analysis of outcome (arterial partial pressure of oxygen) in randomized controlled trials comparing therapy A with therapy B in the treatment of acute exacerbations of chronic obstructive pulmonary disease.
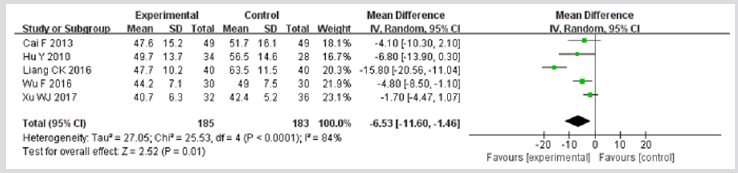
PaCO2: Five studies [20,23,24,29,30] reported the data on change of PaCO2 after treatment. These data were not found to be homogeneous (I2>50%), applying the random effect model in this meta-analysis. As shown in Figure 6, TRQI had an advantage of reducing the level of PaCO2 compared to control group (MD = -6.53; 95%CI, -11.60 to -1.46; p=0.01).
Figure 6: Meta-analysis of outcome (arterial partial pressure of carbon dioxide) in randomized controlled trials comparing therapy A with therapy B in the treatment of acute exacerbations of chronic obstructive pulmonary disease.
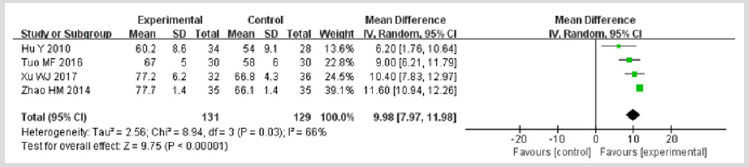
FEV1% Predicted: Four trials 23,28,30,31 provided data for this outcome. Significant heterogeneity was detected among data from the included studies (I2>50%). As illustrated by Figure 7, the results illustrated that compared with therapy B, therapy A obviously improved the FEV1% predicted (MD = 9.98; 95% CI 7.97–11.98; p<0.00001).
Figure 7: Meta-analysis of outcome (forced expiratory volume in 1 second as a percentage of the predicted value) in randomized controlled trials comparing therapy A with therapy B in the treatment of acute exacerbations of chronic obstructive pulmonary disease.
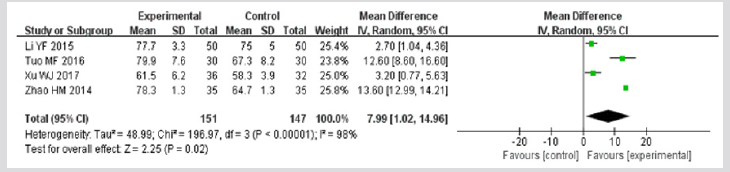
FEV1/FVC: Four trials [26,28,30,31] provided data for this outcome. Significant heterogeneity was detected among data from the included studies (I2>50%). As illustrated by Figure 8, the results illustrated that compared with therapy B, therapy A obviously improved the FEV1/FVC (MD = 7.99; 95% CI 1.02–14.96; p=0.02).
Figure 8: Meta-analysis of outcome (FEV1/FVC) in randomized controlled trials comparing therapy A with therapy B in the treatment of acute exacerbations of chronic obstructive pulmonary disease. FEV1:forced expiratory volume in 1 second; FVC: forced vital capacity.
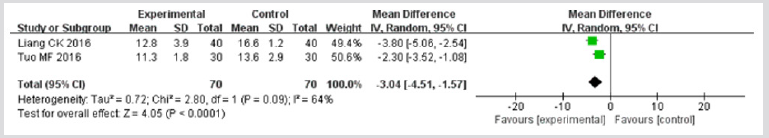
Length of Hospital Stay Only two trials 24,28 involving 140 patients provided data on the length of hospital stay. The I2 test found that the data was not homogeneous (I2>50%), justifying the random effect model in this meta-analysis. A pooled analysis of the two trials showed that therapy A reduced the length of hospital stay to a greater extent than therapy B (MD = -3.04; 95% CI -4.51 to -1.57; p<0.0001) (Figure 9).
Figure 9: Meta-analysis of outcome (length of hospital stays) in randomized controlled trials comparing therapy A with therapy B in the treatment of acute exacerbations of chronic obstructive pulmonary disease.
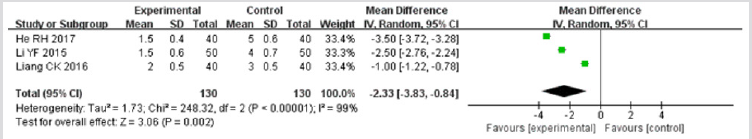
Time to Fever Resolution: Three studies 22,24,26 reported time to fever resolution. The I2 test found that the data was not homogeneous (I2>50%), so the random effect model was used. Meta-analysis showed a significant beneficial effect of TRQI compared to conventional therapy alone in improving the time to fever resolution (MD: = -2.33; 95% CI -3.83 to -0.84; p=0.002) (Figure 10).
Figure 10: Meta-analysis of outcome (time to fever resolution) in randomized controlled trials comparing therapy A with therapy B in the treatment of acute exacerbations of chronic obstructive pulmonary disease.
Figure 11: Meta-analysis of outcome (reduction of cough) in randomized controlled trials comparing therapy A with therapy B in the treatment of acute exacerbations of chronic obstructive pulmonary disease.
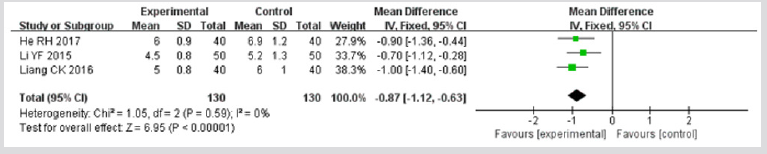
Reduction of Cough: Three studies [22,24,26] reported reduction of cough. It proved to be homogeneous, so the fixed effect model was used. Meta-analysis showed effect of experimental group was superior to control group in improving the reduction of cough (MD: = -0.87; 95% CI -1.12 to -0.63; p<0.00001) (Figure 11).
Duration of Crackles: Three trials [22,24,26] reported the duration for crackles. Meta-analysis (MD: -1.68; 95%CI -1.93 to -1.44; p<0.00001) (Figure 12) about the duration of crackles were conducted. The results showed significant beneficial effects of experimental group compared with control group.
Figure 12: Meta-analysis of outcome (duration of crackles) in randomized controlled trials comparing therapy A with therapy B in the treatment of acute exacerbations of chronic obstructive pulmonary disease.
Adverse Events: Out of 12 included trials three trials [21,23,27] reported adverse reactions including mild facial itching, stomachache and hidrosis. All of these events occurred in patients receiving inhalation of TRQI. After proper medical treatment, all patients experiencing adverse drug reactions completely recovered.
Publication Bias
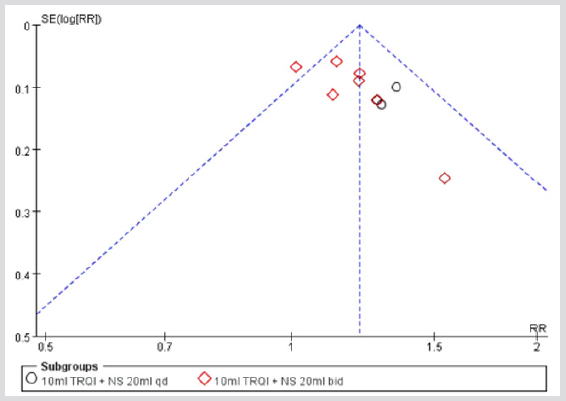
As the most included studies were reporting the clinical effective rates, we chose this data parameter to perform the analysis of publication bias. The funnel plot generated from studies reporting efficacy was asymmetric, suggesting that significant publication bias existed in the included trials (Figure 13).
Discussion
In medicine, systematic reviews and meta-analysis form the core of a movement to ensure that medical treatments are based on the best available empirical data. One important advantage for meta-analysis is that it can enable the user to perform statistical synthesis and then it can be used to enhance the statistical power to obtain a more accurate conclusion [32]. Thus, to systematically evaluate whether aerosol inhalation of TRQI improve PaO2, FEV1% predicted and clinical efficacy, reduce PaCO2, and shorten the length of hospital when combined with western conventional medicine for AECOPD, the authors conducted a systematic review. The results suggested that aerosol inhalation of TRQI intervention in patients of AECOPD may improve PaO2, FEV1% predicted, clinical efficacy, reduce PaCO2, and shorten the length of hospital when compared with western conventional medicine alone. This is the first systematic review of aerosol inhalation of TRQI for AECOPD and the results can provide important references about the efficacy of aerosol inhalation of TRQ injection in the treatment of AECOPD.
In China, it is common to use TRQI by intravenous administration to treat acute and chronic lung or airway diseases in clinical practice [33]. So far, there have been systematic reviews and meta-analysis about TRQI by intravenous administration for community acquired pneumonia, acute exacerbations of chronic obstructive pulmonary disease and acute bronchitis [15,34,35]. However, no systematic review of randomized controlled trials about aerosol inhalation TRQI for AECOPD is published. So, this study may prove useful for supplementing the evidence for the use of aerosol inhalation of TRQI in the treatment of AECOPD.
Tanreqing injection is the combination of water-soluble natural products from five Traditional Chinese Medicines: Radix scutellariae, Flos lonicerae, Fructus forsythiae, Fel ursi, and Cornu saigae tataricae. Of them, Radix scutellariae serves as main agent and the other four ingredients serve as auxiliary medicines. Modern pharmacologic studies have proved that all the five ingredients have the effects of clearing heat, eliminating phlegm, detoxifying, reducing inflammation and alleviating cough and also have antibacterial and antiviral actions [33,36-38]. TRQI is developed from above five components, which suggests that its effect in the treatment of AECOPD may be related with the above pharmacological activities of these TCM. However, what are the specific mechanisms in its effect? Do the interactions between medicines or components exist? These questions are not clear and require further investigation. In this meta-analysis, no significant adverse effects were observed in the included studies, which supported the minimal toxicity and side effects of aerosol inhalation of TRQI. Mild skin allergic reaction or gastrointestinal reaction could be completely relieved after the discontinuation of Tanreqing injection with or without other treatment. This systematic review also has limitations. First, the randomized trials in this review had methodological flaws in terms of allocation concealment and blinding. All the included trials mentioned randomization, while the method about randomization was described in only two trials [26,28]. Allocation concealment and blinding were also rarely described. Selection bias would exist due to lack of reporting random sequence generation and allocation concealment. It was prone to generate measurement bias without blinding method. Second, since all of the included studies on Tanreqing injection were conducted in China, geographic biases may be induced. Third, 9 of the 12 studies were small-scale trials, ranging from 60 to 98 participants per trial. It could lead to exaggerated or weakened results. We also cannot be assured of the safety since the small sample sizes might have limited power to detect rare adverse effects.
Conclusion
This systematic review is the first one that assessed the efficacy and safety of aerosol inhalation of TRQI in the treatment of acute exacerbation of COPD and provided a valuable reference for clinical applications. Within the limitations, we can conclude that compared with conventional western medicine alone, Tanreqing injection administered via aerosol inhalation plus conventional western medicine was of more benefit to patients with acute exacerbations of COPD. However, due to poor quality of the included studies, further large-scale high-quality trials are warranted.
Acknowledgement
We appreciate the research students involved in database search and data extraction.
References
- (2016) Global Strategy for the Diagnosis, Management and Prevention of COPD. Global Initiative for Chronic Obstructive Lung Disease: GOLD.
- Mathers CD, Boerma T, Ma Fat D (2009) Global and regional causes of death. Br Med Bull 92: 7-32.
- Spencer S, Jones PW (2003) Time course of recovery of health status following an infective exacerbation of chronic bronchitis. Thorax 58(7): 589-593.
- Suissa S, Dell'Aniello S, Ernst P (2012) Long-term natural history of chronic obstructive pulmonary disease: severe exacerbations and mortality. Thorax 67(11): 957-963.
- Celli BR, Thomas NE, Anderson JA, Ferguson GT, Jenkins CR, et al. (2008) Effect of pharmacotherapy on rate of decline of lung function in chronic obstructive pulmonary disease: results from the TORCH study. Am J Respir Crit Care Med 178(4): 332-338.
- Ko FW, Chan KP, Hui DS, Goddard JR, Shaw JG, et al. (2016) Acute exacerbation of COPD. Respirology 21(7): 1152-1165.
- Guo R, Pittler MH, Ernst E (2006) Herbal medicines for the treatment of COPD: a systematic review. Eur Respir J 28(2): 330-338.
- Mehta DH, Gardiner PM, Phillips RS, McCarthy EP (2008) Herbal and dietary supplement disclosure to health care providers by individuals with chronic conditions. J Altern Complement Med 14(10): 1263-1269.
- Hu J, Zhang J, Zhao W, Zhang Y, Zhang L (2011) Cochrane systematic reviews of Chinese herbal medicines: an overview. PLoS One 6(12): e28696.
- Chung VC, Wu X, Ma PH, Ho RS, Poon SK, et al. (2016) Chinese Herbal Medicine and Salmeterol and Fluticasone Propionate for Chronic Obstructive Pulmonary Disease: Systematic Review and Network Meta-Analysis. Medicine (Baltimore) 95(20): e3702.
- Liu S, Shergis J, Chen X, Yu X, Guo X, et al. (2014) Chinese herbal medicine (weijing decoction) combined with pharmacotherapy for the treatment of acute exacerbations of chronic obstructive pulmonary disease. Evid Based Complement Alternat Med 2014: 257012.
- Liu SY, Xue DS, Pan JC, Zhang WM, Li WL (2014) Screening and identification of multiple components in Tanreqing injection using RP-HPLC combined with DAD and ESI-TOF/MS. Chin J Nat Med 12(7): 535-541.
- Yang YH, Lai G (2007) Clinical progress on Tanreqing injection in the treatment of respiratory diseases. Chin J Curr Clin Med 5: 491-492.
- Gong G, Li X (2009) Study of clinical effect on treatment of chronic obstructive pulmonary disease in acute aggravated stage with Tanreqing injection and cell factor level. China J of Material Medica 34(1): 104-106.
- Jiang HL, Mao B, Zhong YQ, Yang HM, Fu J (2009) Tanreqing injection for community-acquired pneumonia: A systematic review of randomized evidence. J Chin Integr Med 7(1): 9-19.
- Li M, Zhu L, Liu B, Du L, Jia X, et al. (2016) Tea tree oil nanoemulsions for inhalation therapies of bacterial and fungal pneumonia. Colloids Surf B Bio interfaces 141: 408-416.
- Nahar K, Gupta N, Gauvin R, Absar S, Patel B, et al. (2013) In vitro, in vivo and ex vivo models for studying particle deposition and drug absorption of inhaled pharmaceuticals. Eur J Pharm Sci 49(5): 805-818.
- (2013) Group of Chronic Obstructive Pulmonary Disease, Branch of Respiratory Diseases, and Chinese Medical Association. Guidance of diagnosis and treatment of chronic obstructive pulmonary disease (reversed edition in 2013). Chin J Tuberc Respir Dis 36(4): 255-264.
- Zheng X (2002) ed Guidance for clinical research on New Drugs of TCM. China Medical Science Press.
- Cai F, Zhang QL, Yu ZY, Zhang S, Xiao Z (2013) Tanreqing combined with Terbutaline aerosol inhalation treatment of acute exacerbation of chronic obstructive pulmonary disease. J Intern Med Concepts Pract 8(4): 283-284.
- Dang F (2012) Tanreqing injection atomization treatment curative effect observation of 30 cases of chronic obstructive pulmonary disease. J Emerg Tradit Chin Med 21(8): 1325.
- He R (2017) Tanreqing injection atomization inhalation in the treatment of COPD exacerbations phase analysis of the clinical effect. Henan Med Res 26(9): 1657-1658.
- Hu Y, Yang C (2010) Combining traditional Chinese and western medicine treatment of acute exacerbation of chronic obstructive pulmonary disease curative effect observation. J Pract Tradit Chin Med 26(10): 698-699.
- Liang CK, Ye L, Li S, Zhou ZW, Li J (2016) Tanreqing atomization inhalation combined vibration row phlegm machine acute phase curative effect of chronic obstructive pulmonary disease research. Jilin Med J 37(5): 1157-1159.
- Liu NN, Zhou JZ, Zhang B (2008) The different method curative effect comparison of COPD patients. Clin Med Eng 15(12): 88-89.
- Li YF, Ren HX, Zhang W, Zhuang SN, Wang D, et al. (2015) Tanreqing aerosol inhalation in patients with acute exacerbation of chronic obstructive pulmonary disease period research on the effects of inflammation. Ningxia Med J 37(6): 555-557.
- Saren TY, Li X (2017) Atomization inhalation of Tanreqing observed for the treatment of chronic obstructive pulmonary disease. Clin Res 13: 174-175.
- Tuo MF, Guo LL, Zhao BB, Qiu HL, Wang X (2016) Study on the Clinical Efficacy and Economics of Intravenous Combined with Inhalation of Tanreqing Treatment on Moderate and Severe Acute Exacerbation of Chronic Obstructive Pulmonary Disease. Chin J Mod Appl Pharm 33(4): 484-488.
- Wu F (2016) Tanreqing for the treatment of chronic obstructive pulmonary disease with acute aggravating period of clinical effect. Chin Prac Med 11(28): 223-224.
- Xu WJ, Sun XJ, Ding MG, Xu XW, Zhou C (2017) Tanreqing injection atomization inhalation in treatment of acute exacerbation of chronic obstructive pulmonary disease curative effect observation. J Emerg Tradit Chin Med 26(5): 908-910.
- Zhao HM, Liu HJ, Guo X (2014) Tanreqing atomization inhalation in the treatment of COPD exacerbations period curative effect observation and nursing care. Capit Med 2: 29-30.
- Michael BL, Hedges V, Higgins J (2009) HR : Introduction to Meta-Analysis. Rothstein © John Wiley & Sons, Ltd.
- Wang Y, Wang T, Hu J, Ren C, Lei H, et al. (2011) Anti-biofilm activity of TanReQing, a Traditional Chinese Medicine used for the treatment of acute pneumonia. J Ethnopharmacol 134(1): 165-170.
- Zhong Y, Mao B, Wang G, Fan T, Liu X, et al. (2010) Tanreqing injection combined with conventional Western medicine for acute exacerbations of chronic obstructive pulmonary disease: a systematic review. J Altern Complement Med 16(12): 1309-1319.
- Wang P, Liao X, Xie YM, Chai Y, Li LH (2016) Tanreqing injection for acute bronchitis disease: A systematic review and meta-analysis of randomized controlled trials. Complement Ther Med 25: 143-158.
- Wu T, Yang X, Zeng X, Poole P (2008) Traditional Chinese medicine in the treatment of acute respiratory tract infections. Respir Med 102(8): 1093-1098.
- Yu CM, Zhou AH, Zhang L, Chen J (2013) The Clinical Efficacy of Tanreqing Injection in the Treatment of Children with Acute Lower Respiratory Tract Infection. J Pediatr Pharm 19(8): 25-27.
- Zhu H, Chen M, Shi X, Shi C, Huang C (2017) Material basis studies of anti-Influenza A active ingredients in Tanreqing Injection. Biomed Chromatogr 32(2).

 Research Article
Research Article