Abstract
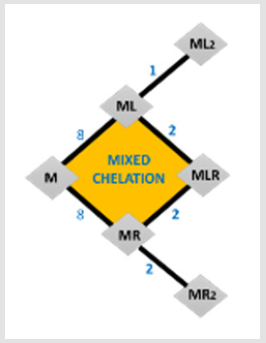
Removal of oversupply of toxic metals has been accomplished medically by the addition of metal chelators. Metal chelators, while useful, have many disadvantages, one of which is the undesired complexation and removal of other essential metals. In the reverse sense, metals are capable of ligand scavenging or mixed complex formation. The degrees to which metal ions can chelate greatly depend on the various chemical factors like stability, solvent nature, ionic strength and statistical ratio etc. In this review, we provide update covering above-said factors, biological importance and recent methods leading chelation (Figure 1)..
Keywords: Chelation; Stability; Ionic Strength; Solvent Effect; Chelating Methods
Introduction
In addition to the diverse applications of chelation in catalysis, material synthesis and photochemistry, chelation displayed in biological systems enables metals to attach with or to move away from the susceptible target owing to to ease or hampering those intracellular connections which might finally lead to cancer [1]. In biological system metal ions usually, form ternary complexes which mainly involve the interaction of the metal ion with two or more different ligands [2]. Recently there has been considerable interest in the mixed chelation because it occurs commonly in biological fluids, which contain millions of potential ligands which are likely to compete for metal ions, found in vivo [3]. It is well known that the ternary coordination complexes play an important role in biological processes as exemplified in many instances by which enzymes are known to be activated by metal ions 4. Ternary complexes containing an amino acid as a secondary ligand have significance as they are potential models for enzyme metal ion substrate complexes Ternary complexes have also been implicated in the storage and transport of active substances through membranes and these phenomena are strongly dependent on the formation of these species and the electronic configuration of metal ion concerned [4]
Biological Importance
The essential metal ions like Cu (II), Co (II), Ni (II), Zn (II), have a significant role in complexation with amino acid and peptides in a living system which acts as a model for many complexes metals-amino acid equilibria occurring in the enzymatic process [5]. Metal chelates having vacant orbitals appear to combine with Sulphur bridge coating of the virus, leaving the DNA and later destruction of protein. All this involves ternary complex formation [6]. The role of cobalt in biological systems is widely investigated in series of coenzyme and vitamin B12. The complexes of cobalt with methionine, lysine and serine play a significant role in bacteriostatic and inhibition of virus replication [7]. Nickel is one of the most important trace elements plays numerous roles in the biology of microorganisms, animals and plants [7]. Zinc plays either a predominantly catalytic role or a solely structural role to maintain the protein configuration. It is a versatile ion as it can bind to different combinations of ligand types resulting in a broad range of stability, reactivity and functions [8]. The chelates formation occurs in biological fluids through transition metals with one or more than one coordination site of ligands of different functional
group has a significant role in detoxification and remediation of metal pollutants [9].Study of Mixed Ligand Metal Complexes
The study of mixed ligand complexes having one synthesized ligand attached to metal ion has received great importance in recent years because of their wide applications in various fields and because of their presence in biological systems [10]. The stability of the mixed ligand complex is measured by the overall formation constant i.e. According to the equilibrium,
M + iL + jB ⇄ MLiBj
Where M is the metal ion, L and B are either neutral or anionic or cationic ligands. If n = i + j ≤ N . Where n cannot exceed the coordination number N, a total of N-n coordination sites will be occupied by solvent molecules and n is the level coordination number when ligands are monodentate [11]. The equilibrium constant for the system involving the formation of a metal complex from the aqua metal ion and the most basic form of the ligand, the stereochemical configuration of the parent complexes is very important. Mixed-ligand complexes are formed only between two suitable ligands, which are not markedly different in their properties. So, in general, no mixed-ligand complex can be formed if reacting species are different in geometrical configurations. There can also be specific reasons such as conjugation effects, stabilization of one of the parent complexes or chelate formation [12].
According to Theory of mixed ligand complexes, the formation of the mixed ligand complexes depends on the nature of the metalligand bond whether σ - or π -, σ - bond affects the mixed-ligand formation because the destabilization caused by ligand repulsion (electrostatic) is of smaller magnitude in mixed-ligand complexes, compared to that in binary complexes [13]. In a system containing one metal ion (Mn+) and two ligands, L and R with similar coordination tendencies lead to complexation simultaneously to form the mixed ligand complex, MLR.
M + N + R ⇄ MLR
β111 = [MLR]/[M][L][R]
If ligand L has a higher coordinating tendency than R, a mixed complex result in two distinctly separate steps.
M + L ⇄ ML
ML + R ⇄ MLR
β111 = [MLR]/[ML][R]
The Mixed Ligand Complexes Are of Two Types
1. Ternary complexes those are more stable than the binary in contrast to the statistical considerations.
2. Ternary complexes those are less stable than the binary complexes, as expected from statistical consideration [14].
The statistical aspect is that the tendency of the metal ion to be bound with the ligands decrease with an increase in the number of bound ligands. However, the values of the formation constant of mixed ligand complexes are observed to be higher or lower than those expected from statistical considerations. This has been attributed to several factors, including that of the electrostatic effect and higher stabilities of the ternary complex, which also result by a direct charge transfer between the two bound ligands with proper orientation. Electron withdrawing substituents lower the stability, whereas electron donating groups increase the stability of the ternary complex [15]. Another explanation quoted in terms of HSAB principle is that, because of back donation of electrons from the d-orbitals of metals to bi-py, the metal ions becomes a harder acid favouring coordination with oxygen donor ligands. John Teller effect has also been considered as an additional factor [16].
ΔlogK is the difference between the stability constant of 1:1:1 ternary complex and corresponding 1:1 binary complex i.e
Δlog k = log KMLR − log KML = orΔlog K = log KMLR − log KMRor Δlog K = log KMLR − (log KML + log KMR) .
Thus, if Δlog K values are positive the ternary complexes are more stable than the corresponding binary complexes and if the values are negative the values of Δlog K do not preclude the formation of ternary complexes in solution.
Following Qualitative Observations Have Been Made About Δlog K For Ternary or Mixed Ligand Complexes:
1. The Δlog K has a negative value when the coordination of the second ligand is through two nitrogen atoms (aliphatic amino acids).
2. The Δlog K value is less negative than the previous case when the secondary ligand coordinates through oxygen and a nitrogen atom (amino acids)
3. The Δlog K value is zero or positive when the secondary ligand coordinates through two oxygen atoms.
Bjerrum has given the classification of factors affecting the stability of mix ligand formation. According to which the factors controlling the stability of mixed ligand complexes are two-fold “statistical” and “ligand effects”. The ligand effect depends upon the electrostatic nature of the M-L bond. The stability constant and complexation behavior of Co (II), Zn (II) and Cu (II) complexes with various ligand has been studied extensively.
Statistical Effects
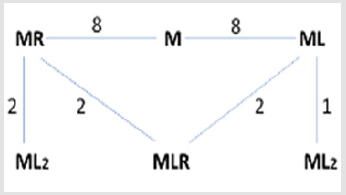
It has been proposed that the mixed ligand complexes involve the consideration of a statistical effect. According to this, if the ligand L and R have equal concentrations, the probability that the first bond ligand L is one half. The probability of the formation of ML3 is, therefore, 1/2 x 1/2 x 1/2 = 1/8. The same probably holds for MR3. The remaining six probabilities are equally divided between the mixed species ML2R and MR2L. The statistical probability of forming either of the latter mixed species is three times that of either simple binary complex.
There are eight different possibilities for the connection of the first ligand in the case of both the bi and tridentate ligands [17] since a tridentate ligand occupies three coordination sites, there is only one possibility for the coordination of a further tridentate ligand, and two possibilities for a bidentate one. If the first ligand to be coordinated is bidentate, then there either are two possibilities for the coordination of a further bidentate or tridentate ligand too. These relations are illustrated in the following scheme, where the tridentate ligand is denoted by R and the bidentate by L (Figure 2).
Effect of Ligand
The evaluation of “ligand effect” expressed as stabilization constant is done by the application of polarized model ion concept. This model treats the complex as a system of the polarized sphere in contact, held together by purely electrostatic forces.
In 1977 the “ligand effect” was calculated by neglecting the polarizability of these spheres, thus, predicting the stabilization of the ternary complex [19]. To account for the stabilization of mixed ligand complexes in some cases, Shelki and Jaghirdar introduced the polarization effect on the calculation of ligand effect by means of point dipoles assumed at the centers of the spheres [18].
Effect of Solvent
The effect of solvent on the formation and stability of mixed ligand complexes has been reported, in several cases, especially in systems where binary and ternary complexes differ in charges. The neutral, mixed - ligand complex formed by the neutralization of charges on the central metal ion and ligands is more stable than the one whose formation does not involve neutralization of charges [19]. The neutralization of charges would result in the positive entropy change due to a decreased orientation of the solvent molecules thus the “charge neutralization effect” may probably be attributed to the favourable entropy changes in the dissolution of the charged species [20]. When both, binary and ternary complexes are neutral, the polar solvent effects may probably stabilize the ternary complex, since the structural difference in the two ligands would induce polar nature in the mixed ligand complexes. This is a result of changes in the polarity of the bonds and the corresponding changes in the energy of intermolecular interaction of electron density occupying formation of mixed ligand complexes.
The Mutual Interaction of The Ligands
It is observed that bond formation between the twocoordination group L and R have also to be considered in the stabilization of mixed species MLR. The ligand interaction may also give a stereoselective effect. The necessary condition for the formation of the MLR is that the two ligands must combine with the metal ion in different pH ranges. The formation of ML should be complete in the lower pH and MLR should be in the higher pH range where the combination of R starts with ML.
Ionic Strength
The stabilization of mixed ligand complexes is considerably affected by the variation in the ionic strength and this fact is of great importance in biological fluids where parameters such as ionic strength and dielectric constants are extremely variable. If the binary and ternary complexes carry equal charges, the stability increases with ionic strength [21].
Redox Potential of The Central Metal Ion
The overall stability of the mixed complex was found to increase with increase in redox potential of the central ion and decrease in the potential of ligand if metal-ligand bond is a predominantly covalent in character. If the bond is ionic, the stability increases with increase in the ligand potential.
General methods for the study of mixed ligand complexes
The equilibrium process can generally describe the formation of mixed ligand complexes. Various modern techniques are used to determine the stability constant of simple as well as mixed ligand compounds.
Potentiometric Method
The principle of the method is that a solution of known concentration of the base (or acid) to be studied is titrated with a strong acid (or strong base) and the reaction is carried out potentiometrically [22]. The method can be used for studying the protonation equilibria of ligands which in the protonated and non-protonated form is sufficiently soluble to form at least 10-3M solutions and which do not decompose during the titration.
Polarographic Method
The polarographic method is used to determine the stability constant by plotting a polarographic curve in the presence and in the absence of substances producing complex formation. A shift in the half-wave potential of a metal ion in solution in the presence of an added ligand (anion or neutral molecules) is indicative of complex formation [23].
Conductance Measurement Method
Werner and others to study metal complexes extensively used this method. In the case of a series of complexes of Co (III) and Pt (IV), Werner assigned the correct formulae based on their molar conductance values measured in freshly prepared dilute solutions. In some cases, the conductance of the solution increased with time due to a chemical change, for example:
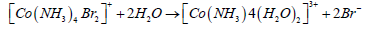
The rise in conductance, in this case, was accompanied by a sharp colour change of the solution from deep green to red.
Spectrophotometric Method
At specific pH, the ratio of the two species (the base and its protonated product) in a solution is determined by spectrophotometry and the protonation constant is calculated by using the basic equation.
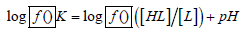
This method can be used in the case of successive complex formation, if the ligand tends to protonate (and the protonation constants are known) and the complex with maximum number of ligands has a selective light absorption at the wavelength of choice while determining stability constants, it is also necessary to know the composition of the complex species formed.
Literature Review
The coordination chemistry of metal complexes plays a vital role in biological system of organism. Transition metal complexes are important in catalysis, material synthesis, photochemistry and biological system. The synthesis of ternary complexes mainly involves the interaction of metal ion with two or more different ligands. Recently there has been considerable interest in the mixed chelation because it occurs commonly in biological fluids, which contain millions of potential ligands which are likely to compete for metal ions, found in vivo. It is well known that the ternary coordination complexes play an important role in biological processes as exemplified by many instances in which enzymes are known to be activated by metal ions [24]. Ternary complexes have also been implicated in the storage and transport of active substances through membranes [25] and these phenomena are strongly dependent on the formation of these species and the electronic configuration of metal ion concerned. The stability constant and complexation behavior of Co (II), Zn (II) and Cu (II) complexes with various ligand has been studied extensively [26,28]. Stability constant and complexation behaviour of divalent metal ion complexes with various ligands has been studied extensively. Some of the examples are included here.
V. Shalini, S. Dharmveer, K. Rajendra in 2015 studied formation of binary and ternary complexes of metal ions such as Cu(II), Co(II), Pb(II), Zn(II) and Cd(II) with biologically important ligands where Adenine (A) was used as primary ligand and amino acid Histidine (B) was used as secondary ligand. Potentiometric technique was applied for determination of complexation behavior of binary and ternary species in aqueous media. Stability constant have been determined through the method suggested by Irving & Rossetti and further refined through SCOGS computer program. The order of overall stability constant of mixed ligand ternary system was determined using the experimental results [29].
H, Hamied in 2015 used a Schiff base ligand synthesized by the condensation reaction of p-phenylenediamine with salicylaldehyde, as a primary ligand and some of amino acid (Alanine or Glycine) as secondary ligand to synthesized complexes containing mixed ligands with the metals ions Co(II), Ni(II), Cu(II) [30]. The prepared ligand(L) and complexes were characterized the compounds were subjected to simultaneous thermogravimetric analysis (TGA/DTA) to study their decomposition mechanism and thermal stability and predicted the geometry of the ternary complexes Wankhede et al. [31] reported the synthesis and characterization of mixed ligand complexes of transition metals such as Mn (II), Fe (III), Co (II), Ni (II), Cu (II) and Zn (II) using Schiff base prepared by condensing salicylaldehyde with ethylenediamine and 8-hydroxyquinoline. The synthesized complexes were characterized and screened for their biological activities such as antibacterial and antifungal. On the basis of the studies made they suggested the octahedral geometry for the complexes.
BD Aghav et al. [32] in 2015 reported the ternary complexes of cerium (III) with 2, 3-dimethyl-1-phenyl-4-salicylidene- 3-pyrazolin-5-one and some amino acids, viz. L-tryptophan, L-tyrosine, L-cysteine, L-leucine and L-serin. These complexes were screened for their antimicrobial activities and show the potent biological activities against Staphylococcus aureus, Corynebacterium diphtheriae, Pseudomonas aeruginosa and Escherichia coli. T. H. Al.Noor , F.H, Ghanim , I. Y. Majeed synthesized new symmetrical Schiff base ligand (H2L) via condensation of hydrazine hydrate and 4-hydroxy-3-methoxybenzaldehyde in ethanol solution at room temperature Polydentate mixed ligand complexes were obtained from 1:1:1 molar ratio reactions with metal ions and H2L, nicotinamide (NA) on reaction with MCl2 .nH2O salt yielded complexes corresponding to the formulas [M(L) (NA)2] . The ligands and their metal complexes were screened for their antimicrobial activity against four bacteria [33].
A. K. Mapari in 2017 examined Binary and ternary complexes of the type M-Y and M-X-Y pH-metrically. The stability constants for Binary (M-Y) and ternary (M-X-Y) systems were calculated [34]. A. A. Al-Rashdi et al. [35] in 2018 reported Binary and ternary complexes of Fe(III), Pb(II), Co(II), Al(III), La(III), Sr(II), Cr(III), Ti(II) and Zr(II) with sulphathiazole (as primary ligand) and amino acid glycine (as secondary ligand) studied potentiometrically. Dharmendra Kumar et al. [36] in 2016 reported the coordination of ternary complexes of biologically active ligands with transition metals. Amino acid asparagine (primary ligand =A) and thiouracil (secondary ligand=B) were applied for complex formation with Cu (II), Ni (II), Zn (II) and Co (II) in aqueous solution (1:1:1). The order of stability of mixed ligands ternary system was determined and the percentage of species formation curves was demonstrated with the help of computer program ORIGIN 6.1 and possible structure of metal complexes with said ligands was discussed. A. Jaleel repoted the syhthesis and characterization of mixed ligand complexes of Co (II), Ni (II), Cu(II), Zn(II) and Cd(II) with (L- lysine )and 8-hydroxyqinoline (Oxine) by : (FT-IR, UV-Vis, AAs) spectra, melting point, molar conductivity measurements [37].
Shatha M. H. O. Al Naimi reported a new Schiff base synthesized from benzaldehyde (C6H5CHO) and O- aminoaniline (o-C6H4(NH2)2. Metal mixed ligand complexes of the Schiff base were prepared from chloride salts of Zn (II), Cd (II) and Hg (II) in ethanol and 8-hydroxyquinoline (8HQ) (C9H7NO) [38]. The synthesis complexes were tested in vitro for antibacterial activity of ligands and metal complexes to the pathogenic bacteria. Akalpita S. Bodkhe et al. [39]. reported Mixed ligand Cu(II) complexes of the type [M(Q)(L)]2H2O using 8-Hydroxy Quinoline (HQ) as a primary ligand and N- and/or O-donor amino acids (HL) such as L-threonine, L-proline, L-hydroxyproline, L-isoleucine and L-serine as secondary ligands. They used the agar cup method and tube dilution method to study the antibacterial activity of the complexes against the pathogenic bacteria S. aureus, C. diphtheriae, P. aeruginosa and E. coli. The results were compared with those of tetracycline, which was screened simultaneously and indicated mild antibacterial activity of the complexes. This brief review of literature reveals that study of mix ligand complexes is a diverse field of research. The physico-chemical properties and unique structural features of these complexes have considerable importance in biological systems. The use of modern spectroscopic as well as electroanalytical techniques for the formation of mix ligand complexes and the computational methods have made it interesting and challenging field of inorganic chemistry.
Conclusion
Chelate formation occurring in biological fluids through transition metals with one or more than one coordination sites of ligands of different functional groups has a significant role in detoxification and remediation of metal pollutants. The stability constants and complexation behavior of divalent transition metals ions with various ligands has been studied extensively. Their unique structural features and factors affecting on them might be seriously considered to reveal further interesting facts about theory and practical applications of mixed ligand complexes.
Acknowledgement
We are thankful to Higher Education Commission of Pakistan, department of biochemistry, faculty of science, university of Jeddah, Saudi Arabia and university of Malaya, Malaysia for their kind support.
References
- Piaggesi A, Låuchli S, Bassetto F, Biedermann T, Marques A, et al. (2018) Advanced therapies in wound management. Journal of wound care 27: S1-S137.
- Marco D, Bombi VB, Mass GG (2006) Electrospray mass spectrometry (ESI‐MS) in the study of metal-ligand solution equilibria. Spectrometry Reviews 25: 347-379.
- Port M, Idee JM, Medina C, Robic C, Sabatou M, et al. (2008) Efficiency, thermodynamic and kinetic stability of marketed gadolinium chelates and their possible clinical consequences: A critical review. Biometals 21: 469-490.
- Kaim W, Schwederski B, Klein A (2013) Bioinorganic Chemistry--Inorganic elements in the chemistry of life: An introduction and guide. John Wiley & Sons.
- Marloye M, Berger G, Gelbcke M, Dufrasne F (2016) A survey of the mechanisms of action of anticancer transition metal complexes. Future medicinal chemistry 8: 2263-2286.
- Nie X, Zhang Z, Wang CH, Fan YS, Meng QY, et al. (2019) Bioconjugate chemistry.
- Shrivastav A, Parrey IR, Hashmi AA (2016) SDRP Journal Of Polymer Science & Technology 1.
- Sakharov DV, Lim C (2005) Zn protein simulations including charge transfer and local polarization effects. Journal of the American Chemical Society 127: 4921-4929.
- Arief VO, Trilestari K, Sunarso J, Indraswati N, Ismadji S (2008) Recent Progress on Biosorption of Heavy Metals from Liquids Using Low Cost Biosorbents: Characterization, Biosorption Parameters and Mechanism Studies. Clean–Soil, Air, Water 36: 937-962.
- Cozzi PG (2004) Synthesis, spectral, 3D molecular modeling and antibacterial studies of dibutyltin (IV) Schiff base complexes derived from substituted isatin and amino acids. Chemical Society Reviews 33: 410-421.
- Zamorano A, Rendon N, Valpuesta JE, Alvarez E, Carmona E (2015) Synthesis and Reactivity toward H2 of (η5-C5Me5) Rh (III) Complexes with Bulky Aminopyridinate Ligands. Inorganic chemistry 54: 6573-6581.
- Yu WW, Wang YA, Peng X (2003) Formation and stability of Size-, Shape-, and Structure-Controlled CdTe Nanocrystals: Ligand effects on monomers and Nanocrystals. Chemistry of materials. 15: 4300-4308.
- Metcalfe RA, Lever ABP (1997) Tetraammineruthenium (II) and -ruthenium (III) Complexes of o-Benzoquinone diimine and their redox series. Inorganic chemistry. 36: 4762-4771.
- Majlesi K, Rezaienejad S (2010) Journal of Chemical & Engineering Data 55: 4491-4498.
- Murgida D, Hildebrandt P, Wei J, He YF, Liu H, et al. (2004) Surface-Enhanced Resonance Raman Spectroscopic and Electrochemical Study of Cytochrome c Bound on Electrodes through Coordination with Pyridinyl-Terminated Self-Assembled Monolayers. The journal of physical chemistry B 108: 2261-2269.
- Halcrow M (2016) The Effect of Ligand Design on Metal Ion Spin State-Lessons from Spin Crossover Complexes. Crystals 6(5): 58.
- Selvaraj P, Santappa M (1977) Equilibirium studies of mixed ligand complexes fo uranyl ion with amino acids and carboxylic acids in aqueous solution. Journal of Inorganic and Nuclear Chemistry 39: 119-122.
- Shelke D, Jahagirdar D (1979) Ternary complexes: Equilibrium study of the mixed complexes of copper ion with carboxylic and phenolic acids in dioxane-water mixtures. Journal of Inorganic and Nuclear Chemistry 41: 925-928.
- Fujita M (1998) Metal-directed self-assembly of two- and three-dimensional synthetic receptors. Chemical Society Reviews 27: 417-425.
- Zhou HX, Pang X (2018) Electrostatic interactions in Protein Structure, Folding, Binding, and Condensation. Chemical reviews 118: 1691-1741.
- Bourbigou OH, Magna L, Morvan D (2010) Effect of Water Content on Crystalline Structure of Ionic Liquids Mixture Pretreated Microcrystalline Cellulose (MCC). Applied catalysis A: General 373: 1-56.
- Pagnanelli F, Mainelli S, Veglio F, Toro L (2003) Heavy metal removal by olive pomace: Biosorbent characterisation and equilibrium modelling. Chemical engineering science 58: 4709-4717.
- Yatsimirskii K (2012) Instability constants of complex compounds. Springer Science & Business Media.
- Sherif EA A, Eldebss TM (2011) Synthesis, spectral characterization, solution equilibria, in vitro antibacterial and cytotoxic activities of Cu (II), Ni (II), Mn (II), Co (II) and Zn (II) complexes with Schiff base derived from 5-bromosalicylaldehyde and 2-aminomethylthiophene. Spectrochimica Acta Part A: Molecular and biomolecular spectroscopy 79: 1803-1814.
- Reddy PR, Rao KS (2006) Ternary nickel (II) complexes as hydrolytic DNA-cleavage agents. Chem Biodivers 3(2): 231-244.
- Siegel H, Inorg J (1975) Nuci Chem. 37: 507.
- Gandolfi C, Bium J, Inorg (1983) Chim Acta. 80: 103.
- Azab HA, Hassan A, Nady AM E, Azkal RS A, Furr M (1993) Chem uonthly chemical. 124: 267.
- Shalini V, Dharmveer S, Rajendra K, Kumar SB, Krishna V (2015) Res J Chem Sci 5(3): 42-48.
- H Al-Hmedawi HA, HH Sarah AA J (2015) Kerbala University 13: 4.
- Dnyaneshwar S, Wankhede (2013) Synthesis and characterization of some biologically active mixed ligand complexes of transition metals such as Cr (III), Mn (II), Fe (III), Co (II), Ni (II), Cu (II) and Zn (II). Der chemica sinica 4(5): 79-85.
- Aghav BD (2015) Synthesis, characterization and antibacterial properties of the ternary complexes of cerium with Schiff base derived from 4-aminoantipyrine and some amino acids. Adv Appl Sci Res 6(12): 37-43.
- Taghreed H, Noor A, Ghanim FH, Majeed IY (2013) Advances in physics theories and applications. 17: 23-33.
- Mapari AK (2017) Stability constants of mixed ligand complexes of transition Metal (II) ions with 1-[(1E)-N-(2,4- dibromophenyl) ethanimidoyl] naphthalen-2-ol and 2- {(E)-[4-chlorophenyl) imino] methyl} phenol. Int J of App Chem 13(2): 211-218.
- Rashdi A, Naggar AH, Farghaly OA, Maouf HA, Ekshiba AA (2018) Potentiometric determination of stability constants of sulphathiazole and glycine-metal complexes. Am J of Ana Chem 9: 99-112.
- kumar d, shankar v (2016) formation constants of transition metal mixed ligand ternary complexes with biologically significant ligands. Chem Sci Trans 5(1): 272-278.
- Ibrahim AA J (2011) D.J.P.S. 7: 67.
- Shatha MH O, Naimi A (2016) Synthesis, Characterization and Antibacterial Activities of Mixed Ligand Complexes of Symmetrical Schiff Base and 8-Hydroxyquinoline with Zn (II), Cd (II) and Hg (II). Synthesis J Pathol Microbiol 1(2): 36-41.
- Akalpita SB, Sunil SP, Manzoor MS (2012) Synthesis, characterization and antibacterial studies on mixed ligand copper complexes with polydentate ligands. Acta poloniae pharmaceutica n Drug Research 69(5): 871-877.

 Review Article
Review Article