Abstract
During the cryopreservation process, bovine sperm cells become vulnerable, losing a large percentage of their motility and viability due to cryo-damages. Previous studies focused on the effect of fructose on other bovine species’ sperm viability indicated that the fructose application in the semen media enhanced the viability and motility of sperm cells. This survey aims to investigate the optimal concentration of fructose in the semen extender which is more effective for maintaining sperm viability after thawing. In the present paper, testicles of nine Hostein-Friesian bulls were recovered from a slaughterhouse, transported to the laboratory where sperm was collected from the epididymis’s tail. Immediately after collection, sperm was evaluated for pH, viability, motility and concentration. Then samples were submitted to cryopreservation process using different concentrations of fructose (5, 10 and 20mM – final concentration). After thawing, sperm was examined as afore described. Before freezing, no statistical differences were observed for the results obtained among bulls.
On average, the pH and the motility were respectively 6.0±0.63 and 4.2±0.1. For the viability, in general, values ranged from 48.1% to 77.8% (X=65.0%±2.9). After thawing, besides no statistical differences were observed among bulls, they have been observed among treatments. When compared to the control, results of viability increased on the concentration of 10mM of fructose from 41.2%±3.7 to 47.0±3.8, respectively for the sperm belonging to the control and the 10mM group, while no significant differences were observed for the motility values between these treatments (2.8±0.2). At the concentration of 20mM, a decrease of sperm viability was observed (38.2%±3.8) when compared with the control (P>0.05). A slight decrease was also observed in the motility, in which values decreased to 2.7±0.1. Results of the present study indicated that adding low concentrations of fructose in the extender (10mM) could improve the viability of the sperm cells after thawing. So, fructose is considered to be an efficient energy source for sperm cells who go through the cryopreservation process.
Keywords: Sperm cells; Bovine; Fructose; Cryopreservation
Introduction
Semen cryopreservation is a well-established procedure used in veterinary and human assisted reproduction technology applications. Over the last 50 years, it was used for genetic improvement of beef and dairy cattle. Moreover, it helps the regulation of venereal diseases and facilitate the management of cattle fertility. Normally semen is recovered from alive animals, but in some situations e.g. when the male succumb the only possibility is recovering semen from the epididymis. This portion of the testicle is usually divided into four anatomical regions: the initial segment, head (caput), body (corpus), and tail (cauda). The epididymis is organized into lobules separated by connective tissue septa that serve not only as internal support for the organ but also as a functional separation which allows for selective expression of genes and proteins within each individual lobule [1,2]. The most important factors for successful semen cryopreservation according to various surveys are, the composition of the extender, the suitable cryoprotectants and the optimal freezing and thawing rates [3]. Spermatozoa are characterized by plasma membrane fluidity and low water content which make them more resistant to cryo-damage compared to other cell types such as oocytes [4].
However, cryo-damage can happen as a consequence of thermal stress due to the change in temperature during cooling, freezing and thawing as well as the osmotic stress caused by addition of high concentrations of cryoprotective agents and by crystallization [5]. This results in protein denaturation, shrinkage and irreversible membrane collapse [6]. Therefore, phospholipids and cryoprotective agents, optimal dilution, equilibration and cooling procedures are required to avoid cold shock so as to reduce crystallization and minimize sperm damage [7]. It is well known that a structurally and biochemically active plasma membrane is required to accomplish the processes of capacitation, acrosome reaction and the oocyte penetration. Semen extender is needed to maintain the sperm motility. Spermatozoa require the proper nutrition for their viability. Fructose is a type of sugar which can be easily metabolized into energy. Its addition, into the extender can be a main source of energy for spermatozoa in the seminal plasma for metabolic processes [8]. The use of sugar such as fructose, sucrose, glucose, trehalose, EDTA or rafinose in semen extender, could increase sperm motility and viability (Aisen; Suwarso, 1999 in Arifiantini). Sugar in semen extender could keep osmotic pressure and energy source on specific levels as a cryoprotectant.
In the present study, we search the effect of different concentration of fructose to sperm viability before and after the cryopreservation process. The sperm is extracted from epididymis tail since it is the only part of the testicles where the mature sperm cells are still alive after the death of the bull. Technology has advanced in a way that makes the cryopreservation of bovine semen, through recovery of epididymal sperm, possible enabling the reproduction of elite bulls that can accidentally die [9]. It is shown that, increased levels of fructose more than normal in spermatozoa could be an alternative energy resource which is used in metabolic process to produce ATP. In this way, sperm motility can be increased, and the viability of spermatozoa can be longer than normal [10]. In certain conditions, fructose acts also as an extra cellular cryoprotectant agent, which helps plasma membrane spermatozoa to remain protected of toxicity during storage [11]. Dilution of the semen for freezing purpose decreases the available source of energy for spermatozoa [12]. As a result, sperm motility is affected by the properties of diluting media [13].
Materials and Methods
Testicles of nine bulls from “Holstein Friesian” breed, aged from three to eight years (4.9 ± 2.8) were collected in the slaughterhouse of Terceira Island, transported to the University of the Azores where epididymis were dissected, their tail isolated, and sperm collected and diluted in Tris medium 5mM fructose at room temperature, according to Cormier and collaborators [14]. After collecting, sperm mass motility was determined by phase-contrast microscopy (×200), on a warm stage at 37°C scored subjectively from 0 (no motion) to 5 (numerous rapid waves) on a scale with steps equal to 1 according to the original method described by [15]. Only semen scored 3 to 5 was evaluated and cryopreserved. In addition, the concentration was calculated in a Neubauer counting chamber after the dilution of 10μl semen to 990μl of water in a final concentration of 400 × 106 spz/ml. Before the cryopreservation procedure the sperm viability was assessed with eosin staining according to [16].
Briefly, on a previously warmed slide semen was mixed with 10μl of the eosin solution, and sperm viability was observed under a phase-contrast microscopy (×200). Extender was based on egg yolk and prepared according to [17], Briefly, the following compounds were added: 375 mM Tris, 124 mM citric acid, 41.6 mM glucose, 9% (v/v) egg yolk and 5% (v/v) glycerol by adjusting the pH to 6.8. After this procedure, extender was separated into 3 flasks, in which fructose was added in a final concentration of 5, 10, and 20 mM. After diluting, for each bull 20 “French” straws (i.d.=1.6 mm, IMV. L’Aigle, France) of 0.25 ml were filled and they were cooled in a styrofoam box in the refrigerator at 4 ºC for 2 hours and frozen in nitrogen vapors according to [18]. The post-thawing semen evaluation was performed using the same methodology as afore described.
Results
In the present study, different concentrations of fructose were employed to evaluate their effect on sperm viability after the cryopreservation, collected in vitro from the epididymal tail of the testicle. On total, sperm of just slaughtered 9 bulls were collected and frozen with 5, 10 and 20 mM of fructose. For results, sperm vigor and pH as well as viable sperm cells were evaluated before and after thawing. More recently, Domeniconi and collaborators [19] postulated that the epididymis seems to be a series of organs placed side by side, in which Luminal acidification is achieved by epithelial cells that have specific roles depending on their location along the epididymis [20]. These cells are significantly more numerous in the cauda epididymis compared with the caput region, suggesting a major acidifying role in the distal region, where spermatozoa are stored [20]. On average, in the present study semen pH ranged from 5.5 to 6.5 (6.0±0.63), with our results being in agreement with those published by Ting and collaborators, (2015), in which mammal’s sperm can range among 5.2 to 8.2. The pH of the extender used as control was 6.0.
The cryopreserved bovine semen generally provides lower fertility rates compared to fresh semen. When comparisons were made based on a similar number of motile sperms, the results of fertility of the frozen semen are inferior to those obtained using fresh semen [6]. The main factors involved in the decrease of fertility are temperature changes during the cryopreservation process, the toxic and osmotic stress depicted by exposure to cryoprotectants, the formation and dissolution of extracellular ice crystals [21], among others. In other hand, after thawing a decrease in sperm motility is observed in all species, when compared with fresh semen. In the present study, sperm motility was on average 4.22 (± 0.07) immediately after the epididymal recovery, decreasing to 2.8 (± 0.2) for the control and the treatment with 10 mM of fructose and 2.7 (± 0.1) in the treatment of 20 mM of fructose. This slightly reduce of the motility can be the result of the detrimental effects on the sperm structure and function during the whole process of sperm conservation such those attributed to damage to the mitochondrial membrane.
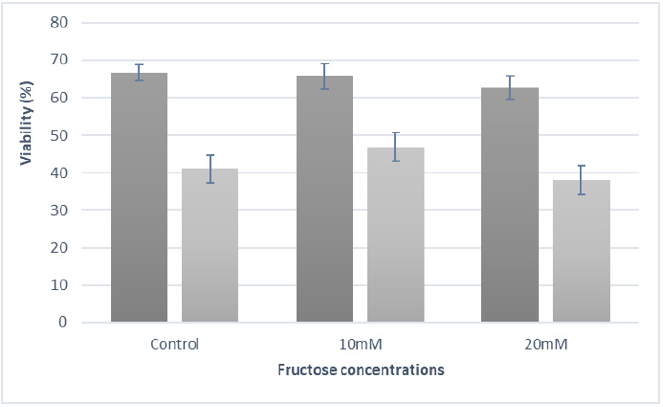
The ATP generated by oxidative phosphorylation in the inner mitochondrial membrane is transferred to the microtubules, to drive motility [22]. Therefore, an impairment of mitochondrial activity may explain the reduction in motility [23]. Although motility is not directly related to the fertilizing capacity, it is normally considered one of the most important factors affecting the sperm quality. Besides a lot of variation can be observed in the susceptibility of an individual donor sperm after cryopreservation, it has been documented that sperm motility decreases by 50% after the freeze thaw cycle [24]. Concerning sperm viability, before cryopreservation, values were on average 65.0% (±2.9), ranging from 66.6% (±2.1), 65.6% (±3.4) and 62.7% (±3.1), respectively for the control, 10 mM and 20 mM. (P>0.05). This value decreased to 41.2% (±3.7); 47.0% (±3.8) and 38.2% (±3.8) (P<0.05). As it can be observed, besides viability decreased after thawing, the loss of viability was much lower in the group where fructose was added at the 10mM final concentration (Figure 1).
Figure 1: Sperm Viability results before and after cryopreservation process with control, 10Mm and 20mM extender.
Discussion
Several explanations can be attributed to these pH, motility and viability results. One can speculate that the increase of fructose at 10mM concentration could act as an energetic source for sperm, reducing their loss of viability after thawing, could be harmful for sperm if the concentration is too high (e.g. 20mM). Despite many hypotheses, the exact molecular mechanisms responsible for decreased sperm fertility during in vitro storage remain unclear [25], yet evidence is accumulating that this reduced fertility is related to the disruption and damage of the sperm membrane [26]. Functional characteristics of spermatozoa are directly affected by the lipid composition of the sperm membrane [7,27,28]. It has been reported that fast cooling in the semen induces lethal stress on some cells, and this is proportional to freezing curve and temperature interval. This process is known as cold shock, and its effects vary according to the species [6].
In general, semen cryopreservation protocols use freezing curves ranging from 10 to 100°C min), resulting in good survival rates after thawing [29]. In bovine semen cryopreservation, glycerol has been frequently used as cryoprotectant in the freezing medium and several studies have shown its efficiency, which, however, is influenced by the freezing curve. Acccording to the pH, studies developed by Carr and Acott [30] indicated that bovine sperm in the caudal epididymis (CE) is maintained in a quiescent state by a pH-dependent inhibitory factor and the motility increases as the sperm pH increases. Thus, it can explain the low pH we got in our study. Moreover, the measured pH can be the result between the epididymal sperm combined with the extender pH, as after dissecting the testicles, epididymis was washed with about 3 ml of extender [31].
Conclusion
In conclusion, this study clearly indicated that spermatozoa obtained from epididymis tail and cryopreserved can use as optimal energy source the fructose, reducing the loss of their viability and motility. As a result, this semen can be further used for reproductive purposes in artificial insemination.
Acknowledgment
This project was financed in 85% by FEDER and in 15% with regional funds through the Programa Operacional Açores 2020 (Operational Program Azores 2020), in scope of the project «BEMAP-ET - ACORES-01-0145-FEDER-000026.
References
- Robaire B, Hinton BT, Orgebin Crist MC (2006) The epididymis. In: Neil J. Knobil’s physiology of reproduction. (4th edn.). Elsevier Academic Press, New York, USA, pp. 1071-1148.
- Turner BL, Kasperson RE, Matson P, McCarthy JJ, Corell RW, et al. (2003) A framework for vulnerability analysis in sustainability science. Proc Nat Acad Sci 100(14): 8074-8079.
- Malo C, Gil L, Gonzalez N, Cano R, de Blas I, et al. (2010) Comparing sugar type supplementation for cryopreservation of boar semen in egg yolkbased extender. Cryobiology 61(1): 17-21.
- Khalil WA, El Harairy MA, Zeidan AEB, Hassan MAE, Mohey Elsaeed O (2018) Evaluation of bull spermatozoa during and after cryopreservation: Structural and ultrastructural insights. Int J Vet Sci Med 6(Supply): 49-56.
- Chantler E, Abraham Peskir JV (2004) Significance of midpiece vesicles and functional integrity of the membranes of human spermatozoa after osmotic stress. Andrologia. 36(2): 87-93.
- Watson PF (2000) The causes of reduced fertility with cryopreserved semen. Anim Reprod Sci 60-61: 481-492.
- Flesch FM, Gadella BM (2000) Dynamics of the mammalian sperm plasma membrane in the process of fertilization. Biochim Biophys Acta 1469(3): 197-235.
- Sansone G, Nastri MJ, Fabbrocini A (2000) Storage of buffalo (Bubalus bubalis) semen. Anim Reprod Sci 62(1-3): 55-76.
- Chaveiro A, Cerqueira C, Silva J, Franco J, Moreira da Silva F (2015) Evaluation of frozen thawed cauda epididymal sperms and in vitro fertilizing potential of bovine sperm collected from the cauda epididymal. Iran J Vet Res 16(2): 188-193.
- Ponglowhapan S, Essén Gustavsson B, Linde Forsberg C (2004) Influence of glucose and fructose in the extender during long-term storage of chilled canine semen. Theriogenology 62(8): 1498-1517.
- Johnson LA, Welch GR, Rens W 1999) The Beltsville sperm sexing technology: high-speed sperm sorting gives improved sperm output for in vitro fertilization and AI. J Anim Sci 2: 213-220.
- Akhter S (2006) Effect of milk-based extenders containing different antibiotics on sperm motility, longevity, morphology, plasma membrane integrity, in vivo fertility and bacterial control of liquid semen from buffalo bulls. Ph.D. thesis, Department of Zoology, University of Arid Agriculture, Rawalpindi, Pakistan.
- Andrabi SM (2009) Factors affecting the quality of cryopreserved buffalo (Bubalus bubalis) bull spermatozoa. Reprod Domest Anim 44(3): 552- 569.
- Cormier N, Sirard MA, Bailey JL (1997) Premature capacitation of bovine spermatozoa is initiated by cryopreservation. J Androl 18(4): 461-468.
- Evans G, Maxwell WMC (1987) Salamon’s artificial insemination of sheep and goats. Tropical Animal Health and Production. Butterworths. Sydney. Australia.
- Burgos MH, di Paola G (1952) Eosin test for the evaluation of sperm vitality. Obstetrical and Gynecological Survey 7(3): 445.
- Atessahin A, Tuncer PB, Bucak MN (2008) Effects of anti-oxidant additives on microscopic and oxidative parameters of Angora goat semen following the freeze-thawing process. Small Ruminant Research 77(1): 38-44.
- Ansari MS, Rakha BA, Akhter S (2017) Cryopreservation of Nili-Ravi Buffalo (Bubalus Bubalis) Semen in AndroMed® Extender in Vitro and in Vivo Evaluation. Reproduction in Domestic Animals 52(6): 992-997.
- Domeniconi RF, Souza AC, Xu B, Washington AM, Hinton BT (2016) Is the Epididymis a Series of Organs Placed Side by Side? Biology of Reproduction. 95(1): 10.
- Shum WW, Ruan YC, Da Silva N, Breton S (2011) Establishment of cellcell cross talk in the epididymis: control of luminal acidification. J Androl 32 (6): 576-586.
- Chaveiro A, Cerqueira C, Silva J, Franco J, Moreira da Silva F (2015) Evaluation of frozen thawed cauda epididymal sperms and in vitro fertilizing potential of bovine sperm collected from the cauda epididymal. Iran J Vet Res 16(2): 188-193.
- Zamboni L (1987) The ultrastructural pathology of the spermatozoon as a cause of infertility: the role of electron microscopy in the evaluation of semen quality. Fertil Steril 48(5): 711-734.
- O’Connell MJ, Bachilo SM, Huffman CB, Moore VC, Strano MS, et al. (2002) Band Gap Fluorescence from Individual Single-Walled Carbon Nanotubes. Science 297(5581): 593-596.
- Keel BA, Webster BW, Roberts DK (1993) Semen cryopreservation methodology and results. In: Barratt C.L.R., Cooke I.D., editors. Donor insemination. Cambridge University Press. Cambridge. UK: p. 71-96.
- Abavisani A, Arshami J, Naserian AA, Sheikholeslami Kandelousi MA, Azizzadeh M (2013) Quality of bovine chilled or frozen-thawed semen after addition of omega-3 fatty acids supplementation to extender. Int J Fertil Steril 7(3): 161-168.
- White IG (1993) Lipids and calcium uptake of sperm in relation to cold shock and preservation: a review. Reproduction, Fertility, and Development. Reprod Fertil Dev 5(6): 639-658.
- Tulsiani DR, Orgebin-Crist MC, Skudlarek MD (1998) Role of luminal fluid glycosyltransferases and glycosidases in the modification of rat sperm plasma membrane glycoproteins during epididymal maturation. J Reprod Fertil Suppl 53: 85-97.
- Lenzi A, Picardo M, Gandini L, Dondero F (1996) Lipids of the sperm plasma membrane: From polyunsaturated fatty acids considered as markers of sperm function to possible scavenger therapy. Hum Reprod Update 2(3): 246-256.
- Forero Gonzalez RA, Celeghini EC, Raphael CF, Andrade AF, Bressan FF, et al. (2012) Effects of bovine sperm cryopreservation using different freezing techniques and cryoprotective agents on plasma, acrosomal and mitochondrial membranes. Andrologia 1: 154-159.
- Acott TS, Carr DW (1984) Inhibition of bovine spermatozoa by caudal epididymal fluid: II. Interaction of pH and a quiescence factor. Biol Reprod 30(4): 926-935.
- Ansari MS, Rakha BA, Andrabi SM, Akhter S (2011) Effect of straw size and thawing time on quality of cryopreserved buffalo (Bubalus bubalis) semen. Reprod Biol 11(1): 49-54.

 Research Article
Research Article