Abstract
We investigated the application and outcomes of a hybride procedure combining left ventricular epicardial lead implantation via mini-thoracotomy with interventional endocardial lead implantation for cardiac resynchronization in the treatment of chronic heart failure. Epicardial lead implantation was applied via a small left-sided chest incision through the fourth or fifth intercostal space in 4 patients with chronic heart failure who were not suitable for left ventricular endocardial lead implantation. The surgical technique of epicardial lead implantation and its short-term outcomes were analyzed. Epicardial lead implantation was successfully performed in all 4 patients, with significant postoperative improvements in hemodynamics, cardiac function and clinical symptoms. 4 Patients were discharged 8, 11, 4 and 7 days after surgery respectively and followed for 12 months. No lead breakage or wound infection were found on followup. The pacing threshold and lead impedance were normal. Phrenic nerve irritation was observed in one patient because the lead was placed lower than that of other three patients but improved obviously after lowering the threshold. No other complications were observed. In conclusion, trans-thoracic left ventricular epicardial lead implantation is a safe, feasible and effective method for cardiac resynchronization therapy. A hybrid procedure combining the interventional endocardial lead implantation with minithoracotomy may maximize CRT outcomes and can be widely applied.
Keywords: Epicardial Lead; Hybrid Procedure; Mini-Thoracotomy; Cardiac Resynchronization Therapy; Congestive Heart Failure; Implantation
Abbreviations:ESC: European Society of Cardiology; LVEDD: Left Ventricular End Diastolic Diameter; LVES: Ventricular End Systolic Diameter; LVEF: Left Ventricular Ejection Fraction; AV: Atrioventricular
Introduction
Chronic heart failure, also known as chronic congestive heart failure, is a commonly seen clinical syndrome. It has gained broad attention from the medical community due to its high morbidity and mortality. Cardiac resynchronization therapy can improve the clinical symptoms of patients with chronic heart failure, enhance their exercise tolerance, and ultimately reduce the rates of hospitalization and mortality significantly. The European Society of Cardiology (ESC) in 2005 and the Chinese Medical Association in 2012 have included cardiac resynchronization therapy in their treatment guidelines as class I indication of non-drug therapy. Patients are unable to undergo transvenous left ventricular lead implantation due to congenital or acquired factors and the incidence rates were 10 to 30% [1]. We performed epicardial left ventricular lead implantation via small chest incisions combining the interventional endocardial lead implantation for CRT in 4 patients with chronic congestive heart failure who were difficult to implantat left ventricular endocardial lead. Here, we present the key technical points, intraoperative status and postoperative follow-up results of these implantation procedures.
Case Report
Case I
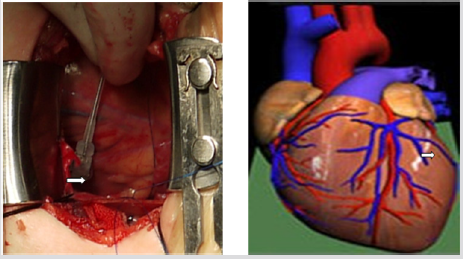
A 76-year-old female patient was admitted due to “repeated episodes of chest tightness and shortness of breath for more than 10 years and chest pain for 1 year, which had been aggravated for half a month.” The patient was diagnosed with dilated cardiomyopathy. Electrocardiography showed sinus rhythm, a QRS length of 170ms, complete left bundle branch block, PR interval of 150 ms, and P wave duration of 110 m s. Echocardiography showed that Left Ventricular End Diastolic Diameter (LVEDD) was 83mm, Left Ventricular End Systolic Diameter (LVES) was 70 mm, and Left Ventricular Ejection Fraction (LVEF) was 26%. The cardiothoracic ratio was 0.70. The clinical characteristics of the case were in accordance with class I indication of CRT. Angiography showed coronary sinus malformation, great cardiac vein and posterior vein of the left ventricle. Hence, transvenous implantation of the left ventricular lead was not possible, and we therefore performed epicardial left ventricular lead implantation via a small chest incision. The surgery was divided into two parts. First, right atrial and right ventricular lead implantation was carried out in a catheterization laboratory. The patient then underwent epicardial left ventricular lead implantation in the operating room. The patient was placed in the supine position and the left chest was elevated. An incision of about 5cm in length was made at the left anterolateral chest (Figure 1). Access to the chest was obtained through the fifth intercostal space. A longitudinal incision was made in the anterior pericardium to expose the heart. Two 6-0 prolene sutures were preset in the epicardium beneath the first diagonal branch of the left anterior descending artery to fix the epicardial lead in the avascular zone (Figure 2). The pacing parameters were measured. The left ventricular impedance was 783Ω, the threshold was 2.0mV, and the R-wave amplitude was 2.5mV. Then, a small incision was made in the corresponding part of the pericardium to allow removal of the epicardial lead line, which was then pulled through the upper intercostal space to the subcutaneous tunnel and brought into the pocket of the pacemaker. A fistula was made in the pericardium posterior to the left phrenic nerve for drainage. Intermittent suture of the pericardial incision was carried out. The pacing parameters were measured again. The left ventricular impedance was 783Ω, the threshold was 1.0V, and the R-wave amplitude was 2.5mV. Phrenic nerve irritation matching the higher threshold was observed once during the adjustment process. A drainage tube was placed through the left chest wall and the chest wound was closed.
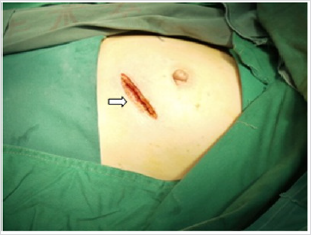
Figure 1: An incision of about 5cm in length was made at the left anterolateral chest through the fifth intercostal space. Comparing with an incision 10cm in length, which can possibly cause relatively massive injury.
Figure 2: An incision of about 5cm in length was made at the left anterolateral chest through the fifth intercostal space. Comparing with an incision 10cm in length, which can possibly cause relatively massive injury.
Case II
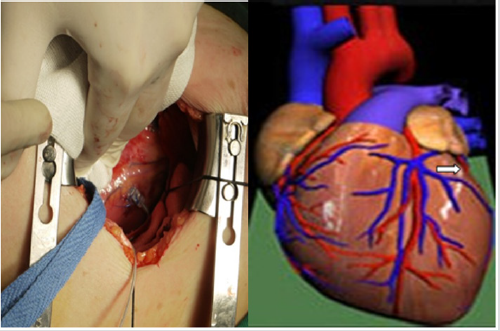
A 59-year-old female was admitted due to “repeated episodes of chest tightness and fatigue for 5 years, concurrent shortness of breath and whole body swelling for 5 months.” The patient had undergone DDD pacing therapy 5 years prior to admission. The clinical diagnosis was dilated cardiomyopathy and third-degree atrioventricular (AV) block. After pacemaker implantation, the cardiac function was still class III. The electrocardiogram showed sinus rhythm and the QRS time was 200ms. Echocardiography showed that LVEDD was 58mm and LVEF was 39%. The cardiothoracic ratio was 0.67. The patient was dependent on ventricular pacing and had cardiac dysfunction, indicating that CRT was necessary for this patient. Subclavian vein angiography showed complete occlusion from the left subclavian vein to the inferior vena cava. Therefore, we performed transthoracic epicardial lead implantation. After general anesthesia, the patient was placed in the supine position and the left chest was elevated. An incision of about 5cm in length was made at the left anterolateral chest (Figure 1). Access to the chest was obtained through the fourth intercostal space using a mini-thoracotomy retractor system. A longitudinal incision was made in the anterior pericardium anterior to the left phrenic nerve to expose the heart. Unlike the first patient, Two 6-0 prolene sutures were preset in the triangle region located inferior to the left atrioventricular groove and superior to the first obtuse marginal branch of the left circumflex artery, and the epicardial lead was fixed in this avascular zone (Figure 3). The pacing parameters were measured. The left ventricular impedance was 640Ω, the threshold was 1.5V, and the R-wave amplitude was 5.0mV. A small incision was made in the corresponding part of the pericardium to remove epicardial lead line, which was then pulled through the upper intercostal space into the subcutaneous tunnel and brought into the pocket of the pacemaker. The pacing parameters were measured again. The left ventricular impedance was 640Ω, the threshold was 1.5V, and the R-wave amplitude was 4.8 mV. A fistula was made in the pericardium posterior to the left phrenic nerve for drainage. Intermittent suture was carried out for the pericardial incision and the chest was closed.
Figure 3: Two 6-0 prolene sutures were preset in the triangle region located inferior to the left atrioventricular groove and superior to the first obtuse marginal branch of the left circumflex artery, and the epicardial lead was fixed in this avascular zone.
Case III
57-year-old female was admitted due to “repeated chest tightness and shortness of breath for more than 6 years.” The patient had undergone three-chamber permanent pacemaker implantation 3 months prior to admission. Clinical diagnosis was dilated cardiomyopathy after CRT implantation with lead micro-dislocation. The cardiac function was class II-III. The electrocardiogram showed sinus rhythm and the QRS time was 198ms. Echocardiography showed that LVEDD was 68mm and LVEF was 35%. The cardiothoracic ratio was 0.66. Because microdislocation of the left ventricular lead was observed during pacing control, and the pacing threshold was high, we performed transthoracic epicardial lead implantation.
The surgical procedure was the same as that described for the second case . The pacing parameters were measured. The left ventricular impedance was 560Ω, the threshold was 1.0V, and the R-wave amplitude was 3.5mV. After the epicardial lead line was removed from the pericardium and placed into the pocket of the pacemaker, the measured left ventricular impedance was 540Ω, the threshold was 1.0V, and the R-wave amplitude was 3.0 mV. A fistula was made in the pericardium posterior to the left phrenic nerve for drainage. Intermittent suture was carried out for the pericardial incision and the chest was closed.
Case IV
A 57-year-old female was admitted due to “repeated episodes of chest tightness and shortness of breath for more than 2 years, which had been aggravated for 1 week.” The patient was diagnosed with dilated cardiomyopathy and complete left bundle branch block. The cardiac function was class III. ECG showed complete left bundle branch block, and the QRS time was 125ms. Echocardiography showed that LVEDD was 75 mm and LVEF was 28%. The cardiothoracic ratio was 0.77. The ejection fraction of non-ischemic dilated cardiomyopathy was equal to or less than 35% and the patient had NYHA class III symptoms. Transvenous implantation of the left ventricular lead was very difficult, therefore, we performed epicardial lead implantation. The surgical procedure was the same as that described for the second case. The pacing parameters were measured after fixing the lead. The left ventricular impedance was 520Ω, the threshold was 0.8 V, and the R-wave amplitude was 4.5 mV. Later, the pacing parameters were measured after the lead line was placed into the pacemaker pocket. The left ventricular impedance was 500Ω, the threshold was 0.9 V, and the R-wave amplitude was 3.5mV.
Surgical Outcomes
Epicardial lead implantation was successfully performed in all 4 patients. The operative times were 135, 60, 70 and 60 min, respectively. There were no serious complications. Enalapril maleate, metoprolol or bisoprolol, furosemide tablets were given after surgery and antibiotics were applied to prevent infection. No other serious complications were observed. Symptoms improved significantly 2 weeks after surgery. Echocardiography showed that the left ventricular end-diastolic diameter reduced from 83mm to 67mm. After the programmable pacemaker was optimized, echocardiography showed improvement in synchronization between interventricular contraction and left ventricular contraction. Left ventricular ejection fraction and cardiothoracic ratio, which were calculated by Simpson’s rule, improved significantly. The patient was discharged 8 days after surgery and no obvious heart failure symptoms were observed. In cases II, III and IV, symptoms such as chest tightness and shortness of breath improved significantly after surgery. The range of blood pressure was 90-98/50-64 mmHg, the heart rate was 60-75/min, and no arrhythmias were noted. No swelling was observed in bilateral extremities and the patients were discharged 11, 4 and 7 days after surgery, respectively. Echocardiography showed that the left ventricular end-diastolic and end-systolic diameters were reduced. LVEF for the 4 patients were 38%, 48%, 43% and 49%, respectively. Moderate to severe mitral regurgitation decreased to mild or moderate regurgitation, and severe tricuspid regurgitation decreased to moderate regurgitation. Ten days after surgery, the threshold of the epicardial lead became lower than 1.5mv, which was stabilized at 0.5-0.8mv during follow-up visits.
Discussion
CRT plays an important role in the treatment of chronic congestive heart failure. It can change the sequence of myocardial activation via left ventricular pacing or biventricular pacing and thereby improve left ventricular ejection function and hemodynamic parameters [2-4]. Many methods have been developed for placing the pacing leads. Among them, transvenous left ventricular lead implantation is the most commonly used method. However, implantation failure occurs due to malformation of the coronary sinus and the target vein, and the incidence is as high as 10%- 30%. Therefore, transthoracic epicardial pacing has become an excellent remedy in clinical practice. Because of the presence of occlusion in the coronary sinus and target vein, four patients in the current study underwent epicardial lead implantation using a transthoracic small incision. Doguet et al. [5,6] reported that the outcome of a reasonable epicardial lead implantation is similar to that of endocardial lead implantation.
Based on our experience, there are two key points in this procedure. First, localization of the left ventricular epicardial lead is very important. In case I, tissue Doppler imaging was applied before surgery, which presented loss of synchronization between the base of the interventricular, septum and the side wall of the left ventricle. The maximum difference value of the time to peak was 251ms, which suggests that the side wall of the left ventricle was the area being excited last. After the lead was placed in the side wall of the left ventricle, Doppler imaging showed that the difference value of the time to peak was only 10 ms, which implies improvement of left ventricular dyssynchrony. In case I, the lead was placed in the epicardium inferior to the first diagonal branch of the left anterior descending artery. The location was relatively lower and the pacing threshold was relatively higher. Moreover, it was close to the phrenic nerve and caused phrenic nerve irritation. Gurevitz [7 ] reported that among 92 patients undergoing CRT, treatment had to be terminated in 4% of patients because of phrenic nerve irritation Biffi et al. [8] reported that the incidence of phrenic nerve irritation is 18%-30% during CRT, which can be prevented by phrenic nerve isolation Champagne et al. [9] found that lead replacement can correct 77% of side effects after the occurrence of phrenic nerve irritation. In clinical practice, methods such as downregulation of the left ventricular output voltage or multi-polar lead placement are used to prevent phrenic nerve irritation [10-12]. In case II, III and IV, we adjusted the location of lead implantation according to the experience of case I. The lead was placed in the triangle zone inferior to the left atrioventricular groove and superior to the first obtuse branch of the left circumflex artery. The pacing threshold decreased compared to case I, and no phrenic nerve irritation occurred. Second, it is critical to reduce surgical trauma and shorten the operative time Shen et al. [13] performed leftsided thoracotomy with an incision 10cm in length, which can possibly cause relatively massive injury. In the current study, we applied a left-sided small incision in the fourth or fifth intercostal space and shortened the length of incision to 5 cm using minimally invasive surgical devices, which provided excellent exposure for easy manipulation with less surgical trauma. Summing up, during cardiac resynchronization therapy, epicardial lead implantation using minimally invasive left-sided thoracotomy is a safe, feasible and effective method. The hybrid surgery combining interventional endocardial lead implantation and mini-thoracotomy can maximize the outcomes of CRT, and is an effective alternative method.
References
- McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Böhm M, et al. (2012) ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012. Eur J Heart Fail 14(8): 803-869.
- Tomczak CR, Paterson I, Haykowsky MJ, Lawrance R, Martellotto A, et al. (2012) Cardiac resynchronization therapy modulation of exercise left ventricular function and pulmonary O2 uptake in heart failure. Am J Physiol Heart Circ Physiol 302(12): 2635-2645.
- Haddad H, Mielniczuk L, Davies RA (2012) Recent advances in the management of chronic heart failure. Curr Opin Cardiol 27(2): 161-168.
- Turschner O, Ritscher G, Simon H, Rittger H, Brachmann J, et al. (2010) Criteria for patient selection in cardiac resynchronization therapy. Future Cardiol 6(6): 871-880.
- Doguet F, Honoré C, Godin B, Anselme F (2012) Isolation of the phrenic nerve to suppress diaphragmatic contraction induced by cardiac resynchronization. J Cardiovasc Electrophysiol 23(7): 778-780.
- Papiashvilli M, Haitov Z, Fuchs T, Bar I (2011) Left ventricular epicardial lead implantation for resynchronisation therapy using a video-assisted thoracoscopic approach. Heart Lung Circ 20(4): 220-222.
- Gurevitz O, Nof E, Carasso S, Luria D, Bar Lev D, et al. (2005) Programmable multiple pacing configurations help to overcome high left ventricular pacing thresholds and avoid phrenic nerve stimulation. Pacing Clin Electrophysiol 28(12): 1255-1259.
- Biffi M, Boriani G (2011) Phrenic stimulation management in CRT patients: Are we there yet? Curr Opin Cardiol 26(1): 12-16.
- Champagne J, Healey JS, Krahn AD, Philippon F, Gurevitz O, et al. (2011) The effect of electronic repositioning on left ventricular pacing and phrenic nerve stimulation. Europace 13(3): 409-415.
- Seifert M, Schau T, Moeller V, Neuss M, Meyhoefer J, et al. (2010) Influence of pacing configurations, body mass index, and position of coronary sinus lead on frequency of phrenic nerve stimulation and pacing thresholds under cardiac resynchronization therapy. Europace 12(7): 961-967.
- Parahuleva MS, Chasan R, Soydan N, Abdallah Y, Neuhof C, et al. (2011) Quadripolar left ventricular lead in a patient with CRT-D does not overcome phrenic nerve stimulation. Clin Med Insights Cardiol 5: 45-47.
- Ohlow MA, Lauer B, Brunelli M, Daralammouri Y, Geller C (2013) The Use of a Quadripolar Left Ventricular Lead Increases Successful Implantation Rates in Patients with Phrenic Nerve Stimulation and/or High Pacing Thresholds Undergoing Cardiac Resynchronisation Therapy with Conventional Bipolar Leads. Indian Pacing Electrophysiol J13(2): 58-65.
- Shen FR, Wang ZJ, Chen JM (2007) The technique of epicardial lead implanted by thoracotomy in cardiac resynchronization therapy. J Electrocardiol 26: 29-31.

 Case Report
Case Report