Abstract
Objective: In this paper, materials are collected on the search for new diagnostic techniques and new generation drugs based on cell technologies that will be used in oncology and in the treatment of cardiovascular diseases.
Materials and Methods: Ex zosomes that are contained in plasma of blood, urine, and other biological fluids may be studied, micro-RNA can be obtained for phenotyping, cell identification and pathological diagnosis.
Results: Exosomes are isolated from hematopoietic stem cells, which can protect damaged cells from death by stimulating cell division renewal, because on the surface of the exotomy, growth antiapoptotic factors that can be used in the treatment of acute ischemic stroke, myocardial infarction, ischemia of the lower extremities, and others severe illnesses. Exosomes can also be used in clinical practice as conveyers for certain types of drugs, delivering them to targeted cells, reducing the possibility of mutations, especially in cancer cases.
Conclusions: Successful introduction of these tools into practical medicine will significantly reduce mortality from these diseases.
Keywords: Exosomes; Intercellular Communication; Target Cells; Oncology; Regeneration; Cardiovascular Diseases
Introduction
One of the most important problems of molecular medicine is the diagnosis and treatment of pathological and oncological diseases. Studies have shown that cells of malignant tumors sometimes “throw” microtubules, releasing tiny particles of RNA, which, when they enter into healthy cells, convert them into cancer. These are extracellular vesicles, whose diameter is 35-90 nanometers, they are divided into intercellular space by cells of various tissues and organs. They are also found in the tissues of the body-in the serum of blood, urine, spinal cord blood and saliva. Their cavity has a cytoplasm and includes various types of proteins, micro-RNAs and lipids. These bubbles have been called “exo-somas” [1]. Of great interest is their potential role in the development of various diseases - from cardiovascular to oncological. First, there are reasons to believe that the appearance of malignant neoplasms significantly increases the amount of exosomes in various biological fluids of man. Second, the analysis of the protein profile and RNA contained in the exosomes may allow judging by their tissue walking and pathological changes in a particular tissue. One can expect that the expansion of the methodical arsenal used today for the study of exosomes will allow for additional information on this new fundamentally biological phenomenon.
Exosomes were first described in 1983 when studying the differentiation of reticulocytes. In 1985, it was demonstrated that exosomes alter the structure of reticulocyte membranes by removing transferrin receptors.
Initially, the exosomes were seen as “reservoirs” - trash cans to remove excess cytoplasm and were perceived as a product of lifeaffluence of cells. In the late 1900s, it was shown that exosomes are involved in the regulation of immune responses in the body, indicating that they play an important role in providing intercellular interaction. In 2007, micro-RNAs and mRNAs were found in the vesicles that transpire genetic information to target cells. This greatly increased the interest in these extracellular vesicles. In the study of cultures of embryonic stem cells, it has been proved that the exosomes are capable of hormonal transfer of mRNA between cells. Exosomes transfer specific mRNAs to the blood cells of the blood, which leads to phenotypic changes in the recipient cells. It is now believed that nucleic acids transmitted by exosomes engage in epigenetic imitation, that is, hereditary changes in the phenotype or expression of genes caused by mechanisms that are not related to the change in the DNA sequence [1].
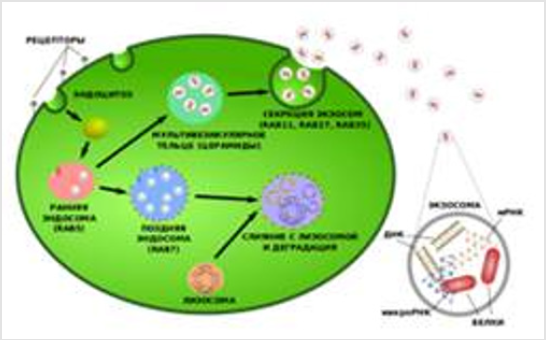
First, the exosomes are formed inside the cell - by the budding of the cavity, called the endosome. When a sufficient amount of exosomes is accumulated in this cavity, their further fraction depends on what lipids are labeled with the membrane of the endosome. If the endosome is labeled with lysobisphosphatidylalcohol (phosphatidylinositol-3-phosphate) and contains ubiquitin proteins, then its contents will be destroyed: it will merge with the lysosome. If the membrane of the endosomes contains ceramides, it will merge with the surface membrane of the cell, and most of the exosomes will escape into the extracellular medium (Figure 1) The path of education is an ecosome the mechanism of absorption by mammalian cells of exosomes is currently poorly understood. This mechanism includes endocytosis, activated by exosome by phosphorylation of extracellular regulated kinase-1/2 (ERK1 / 2), which transmits this signal to the heat shock protein 27 (HSP27). The endocytosis process is negatively controlled by the caveolin-1 protein.
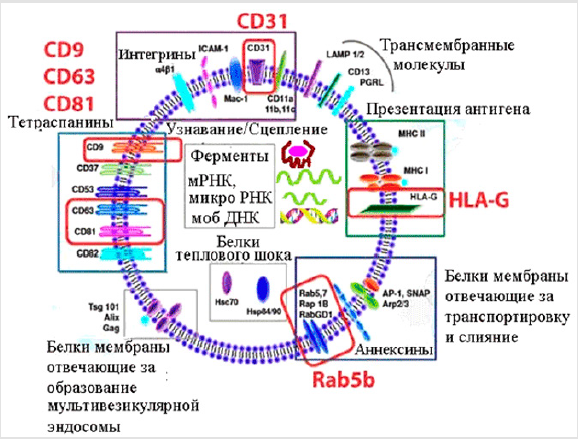
Exosomes include proteins, lipids, micro-RNAs, the content of which necessarily depends on the type of cells that they have isolated, as well as their physiological state.
Exosomes contain the following components:
a) Annexin (Rab5b, AP-1, SNAP, Arp2 / 3) - substances regulating the process of fusion of its membrane with the cell membrane;
b) ESCRT proteins are an endosomal sorting complex that provides an intercellular transfer of proteins and RNA;
c) CD63, CD81, CD9 are transmembrane proteins - exosomal markers;
d) PDCD6IP - an exosomal protein that participates in programmed cell death;
e) membrane proteins of the main complex of histocompatibility of the MHC and stress proteins, or heat shock proteins (HSP60, HSP70, HSP90) [2] (Figure 2).
In an Organism of Man and Animal, Exosomes Perform Various Functions
a) Intercellular Communication
Cells communicate with other cells using exosomes. In some cases, vesicles bind to adjacent cells and isolate micro-RNA in the middle of the cell [2].
b) Immunomodulating Function
Exosomes transport proteins of the main complex of histocompatibility - MHC Class I and II, necessary for the immune response in specialized T-lymphocytes. Also, on their membrane is a special HLA-G receptor, which provides an antigen presentation. NK cells also secrete exosomes. They have substances that, when interacting with a cell membrane (cancer cells), cause its apoptosis [3].
c) Distribution of Viruses and Tumor cells
Tumors and viruses can spread through exosmosis. Vesicles of the tumor inhibit the immune response and provoke metastasis. In addition, they are able to deliver micro-RNA into a healthy cell, thereby changing the expression of genes and disrupting their adequate work.
d) Regenerative Function
Exosomes play an important role in restoring damaged organs. Non-cellular vesicles secreting hematopoietic stem cells with a unique ability: to protect the cells remaining in damaged tissues from death and stimulate their separation. This is due to the fact that the membranes of these vesicles are rich in biologically active lipids, and on the surface of exosomes are synthesized growth and antiapoptotic factors [2]. Materials and methods of extracting exosomes Investigations of exosomes are of great practical importance. For example, they can say a lot about the state of the organism. With exosomes contained in plasma of blood, urine and other biological fluids, micro-RNA can be obtained for diagnosis.
Exosomes contain proteins, micro-RNAs and mRNAs of the cell from which they originate. This allows them to be used for the phenotyping and identification of the cells and the pathological processes that take place there. Of particular importance are exosomes formed in onkotransformovyh-cells. The preparatory stage in the study of exosomes is the use of transplanted cultures of human cells ECV-304 (transformed endothelial cells), glioma-T cells and glioma-B cells (primary glial origin lines). These cells are cultured in a medium DMEM or RPMI-1640 containing 5% embryonal serum without antibiotics in an atmosphere of 5% CO2 at 37°C. As the cells grow, they collect culture-condensed mediumhigh (COP), conduct sequential centrifugation in 2000 and 10000 g for removal from the CS of dead cells and their debris, then the resulting CSF volume of 500 ml is concentrated by ultrafiltration to the final volume 10 ml In the future, the following approaches can be used to distinguish the exosomes from the received preparations of concentrated COP: Ultracentrifugation is carried out on a centrifuge in a mode of 100000 g for 2 hours. After centrifugation, the supernatant fluid is collected in a separate test tube and examined by laser correlation spectroscopy (LKS) to verify the removal of particles of exosomal size from it, the precipitate is dissolved in the maximum volume of phosphate-buffered buffer and re-centrifuged under the same conditions. The resulting precipitate is dissolved in 100 μl of water, broken into aliquots, which freeze at -80°C for further proteomic studies.
Immunosuppression of exosomes with concentrated COs is carried out using immunoaffinity chromatography using antibodies that bind protein markers, often observed on the surface of an exosome. As antigens of exosomal markers, the main type of histocompatibility of the first type (HLA-ABC) is present on the surface of virtually all varieties of cells, and the surface marker of the exosme tetraspanin CD63 is adopted. Monoclonal antibodies to HLA ABC or CD63 (Becman Coulter) are added to of the studied concentrated CS and incubated for 1-2 hours at 4°C, then 100 μl of acefarrose protein solution was added. After 2 hours of incubation at 4°C, the supernatant is collected in a separate tube to control the removal of the exosomal size particles from the COP (using LKS), and the immunoprecipitate is harvested by centrifugation (3000g, 5 minutes at 4°C). The precipitate is washed 2-4 times in 1 ml of phosphate-buffered buffer and centrifuged under the same conditions. The resulting precipitate was dissolved in 100 μl of standard electrophoretic buffer and frozen at -80°C for further proteomic studies.
For Proteomic Studies, the Following Methods are used
a) One-Dimensional Electrophoresis of Exosomes
It is carried out in the presence of SDS with a concentration of 10% polyacrylamide in a separated gel and 5% in a concentrated gel. The analyzed samples are placed in a standard buffer for application (0.065 M Tris, pH 6.8, 2% SDS, 1% DTT, 10% glycerin, 0.01% bromphenol blue) and warmed up in a water bath for 2 min. Samples of protein are applied in an amount of 10-30 μg per track. The concentration of protein in the samples is measured using the Bradford method. Proteins are painted with PAAG after electrophoresis using the Kumasi R350 dye.
b) Two-Dimensional Electrophoresis (2DE)
For 2DE, use the methodological approaches described earlier. Samples containing up to 2 mg of protein are dissolved in 100 μl of lysis buffer (7 M urea, 2 M thiourea, 4% CHAPS, 1% DTT, 2% IPG buffer, pH 3-10, a mixture of protease inhibitors, 0.001% bromphenol blue ) Proteins are separated by isoelectro-focusing using 7-centimeter strips (PH 3.0-10.0). Samples in the lysis buffer (50-500 μg) are mixed with the rehydrating buffer (7 M urea, 2 M thiourea, 2% CHAPS, 0.3% DTT, 2% IPG buffer, the pH range is the same as in the strip, 0.001% bromphenol blue ) in the final volume of 200 μl. In order to prepare strips for separation in the first direction, soak them at 10°C, placing the gel down to the rehydrating solution in a special cuvettes. The isoelectro-focusing is then carried out at 20°C. The strips in front of the second direction are soaked (2x10 min) in a balanced solution (65 mM Tris-HCl, pH 6.8, 6 M urea, 2% SDS and 30% glycerol) containing at first 1% DTT, and then 5% iodoacetamide. Each strip is then placed on top of the gel in a different direction and fixed with a fill of 1 ml of hot solution (0.5%) of agarose containing TGS electrode buffer (25 mM Tris, pH 8.3, 200 mM glycine, and 0.1% SDS).
c) Mass spectrometry
After cleavage with 2DE and Kumassi R350 dyeing, 1-1.5 mm diameter gel bits corresponding to protein stains were cut using microwell tips and partially discolored using a 15-minute incubation in 500 μl of 50% acetonitrile (ACN), containing 25 mM ammonium bicarbonate. The slices are then compressed with a 10-minute incubation in 200 μl of 100% ACN. ACN is removed and the gel is dried for at least 20 minutes at a centrifuge. The dried pieces of gel are soaked for 25 minutes on ice in a 12 μl 25 mM ammonium bicarbonate solution (ABC) containing trypsin. The excess solution is removed, 10 μl of 25 mM ABC solution is added and proteolysis of protein is incubated at 37˚C for at least 4 h. The hydrolysis products are mixed (1 μl) directly on a plate for mass spectrometry with the CHCA (α-cyano-4 hydroxycinnamic acid) matrix dissolved in a concentration of 10 mg / ml in 50% ACN containing 0.1% trifluoroacetic acid, crystallized in the stream air and analyze.
The spectra are obtained in a mode of reflection, using approximately 50-100 laser shots throughout the target area. Total spectra are analyzed using special programs.The result of such an exosomal study is the formation of a protein profile, which allows identification and diagnosis of a particular tissue or organ [4]. Discussion Due to its high specificity, the exosomes can be used in clinical practice as conveyors for certain types of drugs, dostheming them to target cells, reducing the possibility of mutations, especially in cancer cases [5]. In addition, exosomes, which are in the plasma of blood, urine, saliva and other biologic environments, have the opportunity not only to diagnose the disease, but also to establish a stage of the disease that will allow the abandonment of the biopsy. Exosomes are isolated from hematopoietic stem cells that can protect damaged cells from death by stimulating the resumption of cell division, because on the surface of exotomy, growth antiapoptotic factors are synthesized that can be used in the treatment of acute ischemic stroke, myocardial infarction, ischemia of the lower extremities, and other serious diseases.
Conclusion
Thus, despite the fact that the functions of exosomes are at the research stage and have not been fully studied, it is necessary to conclude that with the help of exosomes in the nearest years significant steps will be taken in the diagnosis and therapy of cancer and other serious diseases.
References
- Kuprin AС. The structure and functions of exosomes. The role of exosomes in pathological processes // Youth scientific forum: Natural and medical sciences: electr Sat Art by mat XXIII International stud scientific practice. conf No. 4(22).
- Dzhagarov DE (2013) Exosome - a mechanism for coordination and mutual assistance of the cells of the body. Biomolecule.
- Dzhagarov DE (2013) Exosomes - Bottle Mail of the Organism (Russian). Chemistry and life - XXI century p. 6-9.
- Stam TA, Naryshyn SN, Landa SB, Burdakov VS, Artamonova TO, et al. (2012) Preparation and analysis of exosomes secreted by malignantly transformed human cells in systems in vitro (Russian) // Cytology 54(5): 430-438.
- ЕМ Chevkina, AM Shcherbakov, A Yu Zhuravskaya (2015) Exosomes and transmission (epi) of genetic information by tumor cells // Succession of molecular oncology p. 125-140.

 Perspective
Perspective