Abstract
Objective: This article analyzes the overall management of a patient with advanced non-small cell lung cancer who has been managed for a total of 4 years and provides a reference for clinical case management.
Methods: A Patient with advanced non-small cell lung cancer received multiline treatment including targeted therapy, immunotherapy, anti-vascular therapy, chemotherapy, and radiotherapy. The treatment of the patient during hospitalization was analyzed retrospectively.
Results: Through targeted therapy, immunotherapy, chemotherapy and radiotherapy, the patient’s life span was extended to 4 years.
Conclusion: For advanced lung cancer patients, a comprehensive, Individualized and precise diagnosis and treatment can significantly improve the prognosis of NSCLC.
Keywords: Non-Small Cell Lung Cancer (NSCLC); Targeted Therapy; Immunotherapy; Individualized Treatment
Case Data
The patient was a 64 years old woman, who had no smoking history and no family history of tumor. The blood CEA 304ng/ ml was found in the January 2014 physical examination. PETCT showed right upper lung occupying and multiple lymph node metastases in the right supraclavicular, mediastinal and abdominal pelvis, with multiple small nodules in the upper and the lower lobe of the right lung. Right lung tumor biopsy found low differentiated adenocarcinoma. Molecular Profile was EGFR L858R mutation. According to the imaging and pathological data of this patient, the right lung adenocarcinoma was diagnosed as stage IV, with double lung and abdominal pelvic metastasis.
Treatment Procedures
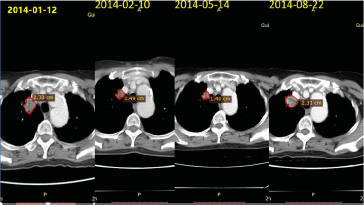
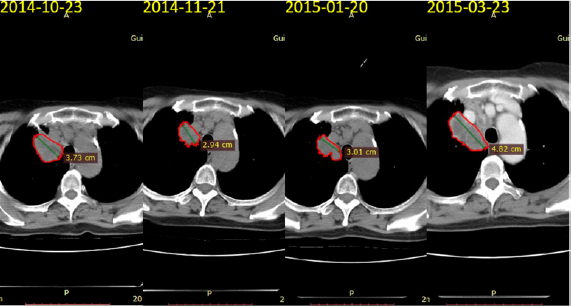
In January 12, 2014, this patient began oral gefitinib as firstline treatment. The tumor gradually reduced, and the blood CEA decreased from 300 to 48.94ng/ml. In August 21, 2014, the CT scan showed that the nodules of the upper lobe of the right lung increased approximately 20%, suggesting progression of the disease. Referring to Matthew K. Wong retrospective analysis of progression of gefitinib first-line treatment, 18 patients received erlotinib as second-line treatment and 12 patients’ disease were under control. So erlotinib second-line treatment was initiated in August 23, 2014. After 2 months (2014-10-23), the chest CT showed that mass continued to increase, which implicated the deterioration of the condition, and the blood CEA value was 27.87ng/ml. After the first generation of TKI drug resistance, we performed molecular testing and no gene mutation was found in October 2014. Blind selection began to take AZD9291 (osimertinib/Tagrisso) as threeline treatment, and the tumor was shrinked (Figures 1 & 2).
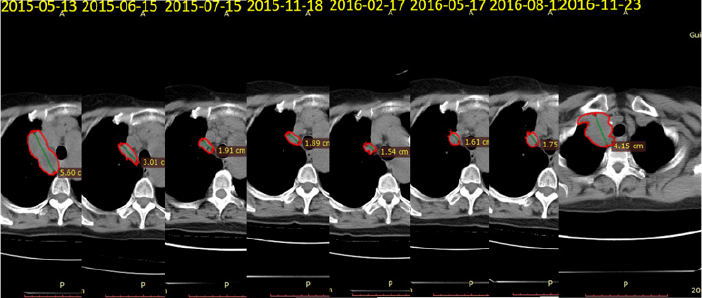
Re-examination of CT in March 30, 2015 showed that lung disease progress and lumbar L1 bone metastasis. So, he received local radiotherapy for lumbar L1 bone metastases. As replacement of pemetrexed+carboplatin chemotherapy as four-line treatment, taxol+carboplatin regimen was performed. The ALTER 0303 study found that PFS and OS were 5.5 months and 9.63 months in the group of NSCLC patients who had previously failed first line and second-line treatment. The PFS and OS in the placebo control group were only 1.4 months and 6.3 months. In May 13, 2015, the patient had the five-line treatment with erlotinib. And after two weeks of treatment, the pain of the whole body was aggravated, and she was unable to take care of herself. The PS score dropped to 3, suggesting that the erlotinib were ineffective. In May 30, 2015, INC280 (c-Met inhibitor)+erlotinib were used as six-line treatment. And 1 day after, the pain significantly relieved, he can have self-care. The best effect was partial response(PR), and the remission period sustained 18 months. In November 23, 2016, the CT showed that lung lesions progressed again, and some new iliac bone metastases appeared (Figure 3).
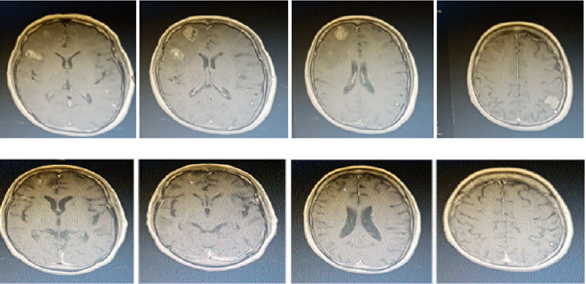
In January 1, 2017, rebiopsy results in Guangdong General Hospital displayed as follow: PDL-1 (SP142) (tumor cells 80%+, immune cells at least 10%+). February 24, 2017 pembrolizumab McAb immunotherapy as seven-line treatment was performed. In 2017-03-23 immune related adverse events (irAEs) showed eyelid swelling with myasthenia, whole body muscle and joint pain, and liver function III disorder. So, he received methylprednisolone + propyl impact treatment, 8 weeks after the patient irAEs, her symptoms relieved. Her antitumor treatments were terminated, and follow-up was observed. 2017-09-07 head MR found multiple intracranial metastases with large edema area. So, He received whole brain radiotherapy and bevacizumab + paclitaxel liposome combined therapy (eight-line treatment) to December 6th, 2017. Seven days after four course treatment, the lung lesions were stable, intracranial multiple lesions and edema basically subsided. Bevacizumab maintenance therapy has been performed until March 1, 2018. The patient’s condition is stable, PS score is 0-1 points, and she could make self-care (Figure 4).
In recent years, with the emergence of various studies on the treatment of advanced NSCLC with epidermal growth factor receptor tyrosine kinase inhibitor (EGFR-TKI) drugs, more and more patients’ life span has been extended . Various guidelines recommend that TKI therapy for advanced NSCLC as first-line, second-line and three-line treatment and even maintenance therapy for advanced NSCLC patient with EGFR mutation. In the course of a certain stage of NSCLC receiving EGFR-TKI treatment, regardless of the short-term curative effect, eventually patients will inevitably have resistance to TKI [1]. Primary drug resistance of EGFR-TKI include T790M mutation, deletion of tumor suppressor gene PTEN or PIK3CA mutation. Abnormal activation of PI3K/AKT pathway, interaction with IGF1R pathway (crosstalk), activation of NF-κB pathway and deletion of Pro apoptotic protein BIM gene polymorphism [2]. Acquired resistance to EGFR-TKI include EGFR second locus T790M mutation, EGFR C797S mutation, C797S and T790M CIS mutation, bypass activation and tissue type transformation.
The tumor cells were resistant to the third generation of EGFR-TKI but were sensitive to the first generation of EGFR-TKI combined with the third generation EGFR-TKI, while C797S and T790M showed a trans mutation. The cells showed resistance to the third generation EGFR-TKI or the first-generation EGFR-TKI, and if C797S appeared in the T790M wild type cells, but the cells will be sensitive to the first generation of EGFR-TKI. The activation of EGFR by-pass includes MET and HER2 amplification, PIK3CA mutation, BRAF mutation and so on. All above is approximately account for 20% of TKI acquired resistance mechanism. Clinical patients have acquired resistance mainly with the following clinical patterns and decisions:
a) Rapid progression. This type of patient recommends having chemotherapy and recommends a prebiopsy.
b) Slow progression. This type of patient recommends TKI treatment, combined with chemotherapy.
c) Locally advanced disease. And these patients recommended to continue TKI combined with local treatment.
Peripheral blood gene detection showed that EGFR 21 exon L858R mutation +c-MET amplification So she was given INC280+AZD9291 treatment. INC280 is an efficient oral c-MET inhibitor, which can be combined with TKI for EGFR mutation and MET positive non-small cell lung cancer. In 2014 ASCO Professor Wu Yilong confirmed that INC280 combined with gefitinib in the treatment of EGFR mutation, MET positive non-small cell lung cancer was effective and well tolerated [3-4]. Tumor immunotherapy aims at activating the body’s immune system and killing cancer cells and tumor tissues by activating T cells. Immunotherapy, as a new type of anti-cancer therapy, has received extensive attention, but in the process of use, it also appears such as immune related rash, immune related pneumonia, hepatitis and thyroid, gastrointestinal tract and other adverse reactions [5], clinicians should pay attention to the adverse reaction of patients and should take appropriate measures in time (Figure 5).
Based on the findings of Keynote-010, FDA approved pembrolizumab monoclonal antibody in October 2015 for advanced NSCLC patients with PD-L1 positive expression. In the KEYNOTE-024 (NCT02142738) study, the first-line application of PD-1 inhibitor Pembrolizumab provides a better progression free survival(PFS) for advanced NSCLC patients with PD-L1 expression of more than 50% (PD-L1 tumor ratio TPS > 50%) and no EGFR or ALK sensitive mutations [6]. In this case, the patients appeared blepharoedema with myasthenia of eyelid, myasthenia of the whole body and joint pain during the treatment of pembrolizumab mAb, the liver function III was abnormal, the treatment of methylprednisolone + propyl ball, and the slow reduction of methylprednisolone, after 8 weeks, the patient’s irAEs was close to be cured, and the curative effect was partly remission. About 10%-15% lung cancer patients have brain metastases at initial diagnosis. Brain metastases occur in the whole course of the disease, and brain metastases are more likely to occur in patients with EGFR gene sensitive mutations.
The median survival time of brain metastases from lung cancer was only 1-2 months. The median OS of whole brain radiotherapy was only 3.5-5.25 months. With the prolongation of the survival time of patients with NSCLC brain metastases, we must pay attention to the neurological impairment caused by whole brain radiotherapy. Targeted drugs emerge in endlessly, PD-1 antibody immunotherapy is advancing rapidly, and more and more methods are used to treat brain metastases. It is questionable to still use WBRT as the only standard treatment for brain metastasis [7]. After 3 years of target and immunotherapy, the patient had multiple brain metastases, and she received whole brain radiotherapy, chemotherapy and anti-vascular treatment, after these treatments, the intracranial tumor and edema got subsided, brain metastases was in remission, PFS obtained further prolonging, whole brain radiotherapy for brain metastases, which means the whole brain radiotherapy still exerting a certain effect. It can’t be replaced by SRS, especially with multiple and larger metastases, local combined with systemic therapy can achieve significant therapeutic effects [8].
Acknowledgment
This work was supported by the grant no. 81660435 and 81860468 from the National Natural Science Foundation of China.
References
- Markakis J (2004) Pastoralism on the margin. Report Minority Rights Group International p. 15.
- Nori M (2005) Sustainable camel milk production and commercialization in Somalia. Future Livestock Systems APS course, Wageningen-4.
- Rhodes S, Crawshaw T, DelaRea Demenech R, Bradford S, Lyashchenko KP (2015) Mycobacterial Infections in Camelids. Tuberculosis, Leprosy and Mycobacterial Diseases of Man and Animals pp. 216-234.
- Mustafa IE (2013) Bacterial diseases of dromedaries and bacterian camels. Rev Sci tech Off Int Epiz 6(2): 391-405.
- Neill SD, Cassidy J, Hanna J, Mackie DP, Pollock JA, et al. (1994) Detection of Mycobacterium bovis infection in skin test-negative cattle with an assay for bovine interferon- gamma. Vet Rec 135(6): 134-135.
- (2012) Oromiya Pastoralist Areas Development Commision (OPADC) Camel Development road map, Oromiya National Regional State, Draft document, Zeway.
- Radostits OM, Gay CC, Blood DC, Hinchclif KW (2007) Veterinary medicine. A text book of the disease of cattle, sheep, pigs, goats and horses. 10th (edn.). London Saunder Elsevier p. 471-500.
- Elmossalami E, Siam MA, El Sergany M (1971) Studies on tuberculosislike lesions in slaughtered camels. Zentralbl Veterinarmed B 18(4): 253- 261.
- Mamo G, Bayleyegn G, Sisay T, Legesse M, Medhin G, et al. (2011) Pathology of Camel Tuberculosis and Molecular Characterization of its Causative Agents in Patoral Regions of Ethiopia. PLoS ONE, A peer- Reviewed Open Access Journal 6(1): 1-10.
- Zubair R, Khan AMZ, Sabri MA (2004) Pathology in camel lungs. J camel science 1: 103-106.
- Abubakar UB, Kudi AC, Abdulkadir IA, Okaiyeto SO (2014) Prevalence of tuberculosis in slaughtered camels (Camelus dromedaries) at Kano abattoir, Nigeria, based on lateral flow technology. Journal of Camel Practice and Research 21(1): 41-45.
- Beye AF, Zerom KG, Mussa A, Ameni G, Sanni MA (2014) Prevalence of bovine tuberculosis in dromedary camels and awareness of pastoralists about its zoonotic importance in Eastern Ethiopia. Journal of Veterinary Medicine and Animal Health 6(4): 109-115.
- Gumi B, Schelling E, Erenso G, Firdessa R, Biffa D, et al. (2012) Low Prevalence of bovine tuberculosis in Somali pastoral livestock, Southeast Ethiopia. Trop Anim Health Prod 44(7): 1445-1450.
- Kassaye S, Molla W, Ameni G (2013) Prevalence of Camel Tuberculosis at Akaki abattoir in Addis Ababa, Ethiopia. African Journal of Microbiology Research 7(20): 2184-2189.
- Mamo G, Kassaye A, Sanni M, Ameni G (2009) A cross sectional study of Camel Tuberculosis in Ethiopia. Bull Anim Hlth Prod Afr 57(1): 13-20.
- Zerom K, Sisay T, Mamo G, Bayu Y, Ameni G (2013) Tuberculosis in dromedaries in Eastern Ethiopia: Abattoir-based prevalence and molecular typing of its causative agents in camels. Research J 109: 188- 192.
- (2008) CSA. Federal Democratic Republic of Ethiopia, Central Statistical Agency. Agricultural Sample Survey Report on Livestock and Livestock Characteristics, Addis Ababa, Statistical bull 2: 16.
- (2012) Central Australian Camel Industry Association Inc (CACIA).
- Schwartz HJ, Dioli M (1992) The one humped camel in eastern Africa. Apictorial guide to disease, Health care and management. Weikeersheini, Verlagiosef monograph p. 1-59.
- Thrusfield M (2007) Veterinary Epidemiology. 3rd (edn.). Oxford, UK: Blackwell Science Ltd, a Blackwell publishing company pp. 228-242.
- Corner LA (1994) Post mortem diagnosis of Mycobacterium bovis infection in cattle. Vet Microbiol 40(1-2): 53-63.
- Bogale B, Woldesenbet Z, Yimer E, Lemma E (2004) Evaluation of abattoir inspection for the diagnosis of Mycobacterium bovis infection in cattle at Addis Ababa abattoir. Trop Anim Health Prod 36(6): 537-546.
- Kinne J, Johnson B, Jahans KL, Smith NH, Ul Haq A, et al. (2006) Camel Tuberculosis-a case report. Trop Anim Health Prod 38(3): 207-213.
- Ameni G, Aseffa A, Engers H, Young D, Hewinson G, et al. (2006) Cattle husbandry in Ethiopia is a predominant factor affecting the pathology of bovine tuberculosis and gamma interferon responses to mycobacterial antigens. Clin Vaccine Immunol 13(9): 1030-1036.
- Vordermeier HM, Chambers MA, Cockle PJ, Whelan AO, Simmons J, et al. (2002) Correlation of ESAT-6- Specific Gamma Interferon with Pathology in Cattle Following Mycobacterium bovis BCG Vaccination against Experimental Bovine Tuberculosis. Infect Immunol 70(6): 3026- 3032.
- Mason FE (1917) Tuberculosis in camels. J comp Path Therap 30: 80-84.
- Manal MY, Gobran RA (2008) Some studies on Tuberculosis in Camel. Egypt J Comp Path and Clinic Path 21(4): 58-74.
- Cleaveland S, Shaw DJ, Mfinanga SG, Shirima G, Kazwala RR, et al. (2007) Mycobacterium bovis in rural Tanzania: Risk Factors for Infection in Human and Cattle Populations. Tuberculosis 87(1): 30-43.
- Inangolet FO, Biffa D, Oloya J, Opuda Asibo J, Skjerve E (2008) A Cross- Sectional Study of Bovine Tuberculosis in the Transhumant and Agro- Pastoral Cattle Herds in the Border Areas of Katakwi and Moroto Districts, Uganda. Trop Anim Hlth Prod 40(7): 501-508.
- Kazwala RR, Kambarage DM, Daborn CJ, Nyange J, Jiwa SF, et al. (2001) Risk factors associated with the occurrence of bovine tuberculosis in cattle in the Southern Highlands of Tanzania. Vet Res Commun 25(8): 609-614.
- Munyeme M, Muma JB, Samui KL, Skjerve E, Nambota AM, et al. (2008) Prevalence of bovine tuberculosis and animal level risk factors for indigenous cattle under different grazing strategies in the livestock/ wildlife interface areas of Zambia. Trop Anim Health Prod 41(3): 345- 352.
- Munyeme M, Muma JB, Skjerve E, Nambota AM, Phiri IG, et al. (2008) Risk Factors Associated with Bovine Tuberculosis in Traditional Cattle of the Livestock/Wildlife Interface Areas in the Kafue Basin of Zambia. Prev Vet Med 85(3-4): 317-328.
- Tsegaye W, Aseffa A, Mache A, Mengistu Y, Berg S, et al. (2010) Conventional and Molecular Epidemiology of Bovine Tuberculosis in Dairy Farms in Addis Ababa City, the Capital of Ethiopia. Intern J Appl Res Vet Med 8(2): 143-151.
- Corner LA, Melville L, McCubbin K, Small KJ, McCormick BS, et al. (1990) Efficiency of inspection procedures for detection of tuberculous lesions in cattle. Aust Vet J 67(11): 389-392.
- Ameni G, Aseffa A, Engers H, Young D, Gordon S, et al. (2007) High prevalence and increased severity of pathology of bovine tuberculosis in Holsteins compared to zebu breeds under field cattle husbandry in central Ethiopia. Clin Vaccine Immunol 14(10): 1356-1361.
- Mason FE (1912) Some observations on tuberculosis in camels in Egypt. J comp Path Therap 25: 109-111.

 Case Report
Case Report