Abstract
Madin-Darby bovine kidney cells, which are known to be responsive to the effect of dioxin, have to been used to investigate if exposure to the 2,3,7,8- tetrachlorodibenzo- p-dioxin could alter their membrane currents. Herein, we present preliminary results showing that this chemical modifies ionic membrane currents and that the modification mainly involves the chloride current component.
Keywords: MDBK; Voltage-gated ion channels; TCDD; Whole cell patch-clamp
Introduction
The name ”dioxins” is used to indicate a great family of structurally and chemically related Polychlorinated-Dibenzo-P-Dioxins (PCDDs) and Polychlorinated-Dibenzofurans (PCDFs). They are environmental toxins of current interest [1] as they can bio-accumulate in food chain, due to their lipophilic nature. The most toxic of these compounds is the 2,3,7,8-Tetrachlorodibenzo-P-Dioxin (TCDD). This chemical, commonly known as dioxin, can cause a wide range of tissue- and species-specific toxic effects, as chloracne, liver damage, disruption of hormone signaling pathways, reproductive and developmental defects [2-4]. Indeed, dioxin can alter brain development, produce cognitive disability, motor dysfunction [5,6], and also induce important alterations in neurodevelopment of human newborns following gestational exposure [7]. Moreover, TCDD may provoke immunosuppression and leads to an increased susceptibility to infectious agents [4,8]. Although the molecular mechanisms for carcinogenicity by dioxin have not been clarified, in 1997 TCDD was classified as a cancer promoter by the International Agency for Research on Cancer [9].
In vitro studies have shown TCDD neurotoxicity in mouse cerebellar granule cells, cortical neurons [10,11] and in NGF- differen tiated PC12 cells [12]. The toxicity of TCDD has been attributed to different mechanisms. In vertebrates, the main molecular mechanism of action responsible of TCDD biochemical effects is the AhR stimulation. Through Aryl Hydrocarbon Receptor Nuclear Translocator (ARNT), the AhR/ARNT complex translocates into nucleus where it promotes various AhR-responsive genes activating their transcription [13,14]. In human SH-SY5Y neuronal cell line, Morales- Hernandez et al. [15] have related TCDD toxicity to a disruption of calcium homeostasis while others [16-18] have established that the Aryl Hydrocarbon Receptor (AhR), to which TCDD binds with a high affinity, is involved in mediating the diverse toxic responses of this dioxin. In Madin-Darby Bovine Kidney (MDBK), an epithelial cell line, through AhR [19,20], TCDD induced a significant cell proliferation [21], increased the incidence cell death with autophagy [19,20,22] and promoted the Bovine Herpesvirus-1 (BoHV- 1) infection as well as BoHV-1-induced apoptosis [4,19-21,23-25]. However, data from literature are sometimes contrasting. For example, the effect on apoptosis signaling can result, dependent on the cell contest, either inducing [19,20] and repressing [17].
Very few are the studies that report effects on the membrane electrical activity after TCDD exposure [26]. The membrane electrical activity is due to the influx/efflux of ions across ion channels and pumps. It regulates the homeosta- sis of the cell [27] and many fundamental processes like as autophagy [28] and apoptosis [29] that also result to be very sensitive to TCDD [4,19-25].
In the present study we used MDBK, a cell line affected by TCDD as defined by our previous studies [4,19-25]. By using a patchclamp technique in the whole cell configuration, we have tested the possibility that the effect of this chemical can alter the normal ionic membrane currents. The preliminary results presented herein show that our cell line undergoes to significant variation of the ionic membrane currents when cultured in the presence of TCDD. Moreover, these variations are largely depending on the increase of the chloride component (Table 1).
Materials and Methods
Cell Cultures
MDBK cells were grown in Dulbeccojs Modified Eagle Medium (DMEM), Glu- taMAX Supplement (Gibco) added with 15% Fetal Bovine Serum (FBS, Gibco) and 1% antibiotic solution containing 10, 000U/ml of penicillin and 10, 000μg/mL of streptomycin (Gibco). The cells were counted using a Burker chamber, seeded (∼ 3 × 104 cells) on 8 mm diameter dishes placed in 35mm2 Petri dishes (Sarstedt) and incubated at 37°C (5% CO2). TCDD (Supelco, 48599) was initially diluted to give a 10, 000pg/ml stock solution by mixing with DMEM. This stock solution was then diluted to give a working solution of 1pg/ml in DMEM, which was added to cultures [4,19-25,30].
Electrophysiological Recordings
Experiments were performed by using an inverted microscope (Nikon, Diaphot 300) mounted with a recording chamber (∼1 ml). Electrodes were prepared pulling thick-wall borosilicate glass pipettes (Clark Electrical Instruments) by using a P1000 micropipette puller (Sutter Instruments). The tip resistance ranged from 2-5M Ω. Patch-clamp recordings in whole cell configuration were performed by an EPC7 amplifier (HEKA Elektronik), using pClamp 8 software and Digidata 1200A for aquisition. Sampling was 10kHz and filtering was 3 kHz. Data were analyzed with pClamp 10. Further analysis has been performed by Qtiplot and Statview. The protocol stimulation (both for treated and untreated groups) was applied starting from a holding level of -80mV and consisted of depolarizing steps (duration 100ms) of amplitude of 10mV from -80 to 100mV (steps separated by 1s). Series resistance and capacitance were compensated.
Preliminary recordings were made in both groups by correcting for the leakage current. These preliminary results have shown no fast current activation. We have consequently, recorded without correction for leakage, and maximal values were considered for times 20ms greater than the stimulus onset. Four different couples (intra- and extracellular) of solutions (A,B,C and D) with different ionic composition were used for both groups. Solution A (basic) con- tained the classical ionic composition for whole cell patch clamp experiments (in- tracellular: 130KCl, 5NaCl, 1CaCl2, 2MgCl2, 2Mg2-ATP, 0.5Na3-GTP, 10EGTA, 10HEPES; extracellular: 135NaCl, 5KCl, 2CaCl2, 1MgCl2, 10Glucose, 10HEPES).
Solution B was the same of A but K+ free (KCl substituted by CsCl). Solution C was Na+ free (NaCl substituted by CsCl) and D was K+Na+ free. The comparison of results obtained using solutions with different ionic composition permitted to understand the ionic contribution to the total currents. To avoid the dependence of the current maximal from the cell size, we normalized the currents to the cell capacitance (current density). The results are then expressed in terms of pA/pF. All results are expressed as means ± SD. ANOVA and post-hoc Games-Howell test were used for statistical significance of differences among the solutions.
Results
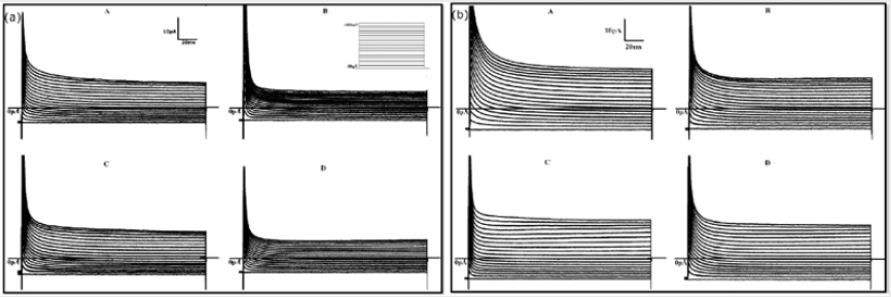
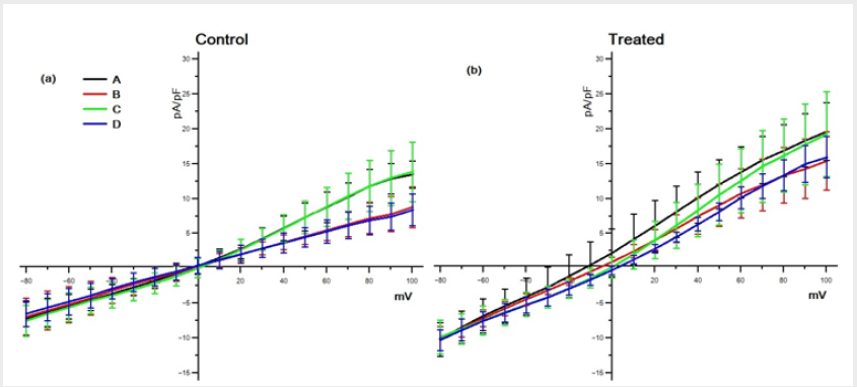
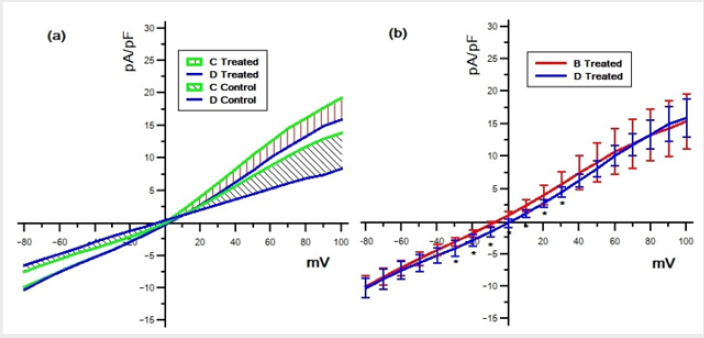
We analyzed 4 groups of cells for the control (n = 13, n = 10, n = 13, n = 10) and 4 groups for the treated (n = 13, n = 12, n = 13, n = 15) with different solutions (respectively with the solutions A,B,C and D). The average density currents (pA/pF) for both groups are shown in Figure 1, control (a) and treated (b). The most evident result shown in this figure is that TCDD treatment enhances the membrane currents for almost all steps and with all solutions used. This effect becomes more evident by looking at Figure 2 where the respective IV for both control (a) and treated (b) groups are shown. It has to be noted that, in the control group (a), the responses with the solutions B and D always remain unchanged (p < 0.05) for all the range of membrane values ( 80mV to 100mV ) and the curves present a linearly increasing trend (slope of < 10-2 and an error on the slope of < 10-4). In the same way, the responses obtained with solution C (Na+ free) does not differ (p < 0.05) from those obtained with solutions A (containing all the ions) but for these curves (slope of < 10-1 and an error on the slope of < 10-3) the linearity is lost for values above 30mV.
Figure 1: Mean total density currents for both control (a) and treated (b) groups registered with the solutions A,B,C and D.
Figure 2: Voltage-current relationships (I/V) obtained averaging the density current at each membrane potential (see Materials and Methods section) in both control (a) and treated (b) cells for all solution groups.
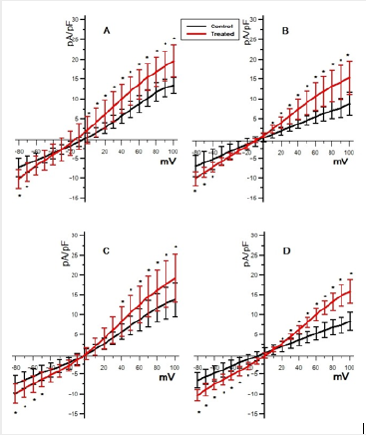
Only at membrane values below 0mV the responses remain unchanged (p < 0.05) with all solutions used. The curves in the panel (b) of Figure 2, although roughly resembling the same shape of the control ones, always present larger density currents. Also, in this case, for positive membrane values the curve C loses its linearity. In addition, for values ranging from -30mV to +30mV, the curves present a more complex combination of the different responses. The results obtained by the solution C (Na+−free) in fact, significantly (p < 0.05) differ from the total and, similarly, those obtained by solutions B (K+−free), significantly (p < 0.05) differ from D (Na+K+−free). The differences between the two groups (a) and (b) are stressed in Figure 3, where the comparison between control and treated data is shown for the same solution. As can be seen the differences are always statistically significant (p < 0.05).
Discussion
As shown in our results, the mostly evident effect of TCDD treatment (1pg/ml) on MDBK cells is to increase the total membrane density currents. Several other observations can be made mostly looking at Figure 4. In the control group for the values below 0mV the non-significant difference be- tween the results obtained with the different solutions groups, indicates that the produced current is due to Cl− ions. In fact, the Na+K+ free solution (D) has only Cl− ions, and this indicates that other ions do not contribute. This assumption is also supported by the fact that all curves invert polarity at 0mV which the reverse potential of our solutions couple for is Cl−. The linearity of results obtained with solution D (Figure 3) indicates that this contribution is probably due to an Ohmic (non-voltage-dependent) conductance. Essentially the membrane behaves like a resistor obeying to the Ohm’s low and giving a current directly proportional to the voltage level. This holds both for control and treated group.
Another observation by considering the results of solution C (green line in Figures 2 & 3) is that a K+ voltage dependent contribution is added for values greater than 0mV both in the control and treated group. In fact, only for the positive voltage steps, the results obtained with this solution significantly diverge from those obtained with solution D. However, the difference between the results obtained with the solutions C and D is significantly larger (p < 0.05) in the control with respect to the treated group, (panel (a) in Figure 4). This could suggest that, following the TCDD treatment, the Cl− component grows more than K+ component, which could instead diminish. The curve B (K+ −f ree) shows a significant (p < 0.05) difference in the treated group respect the control one (Figure 2) and, only in the treated group, it significantly diverges (p < 0.05) from results obtained with solution D for values of membrane potential ranging -30mV to 30mV , see Figure 4b. This difference become not significant for values of membrane potential close to Na+ equilibrium potential (+80mV for our solutions). The lack of significance while the membrane potential is approaching the Na+ reverse potential, indicates that this component could be contributed by Na+ ions. However, the extremely positive value of the reverse potential for Na+ suggests that a current contributed by this ion should be an inward current but the comparison in Figure 4b shows that the contribution is of an outward current. This finding is not easy to explain. At present stage we can only hypothesize a possible mechanism. TCDD treatment could induce the appearance of a possible Na+ current by a possible expression of newly formed Na+ channels, not present in the control conditions. These ionic channels should be voltage dependent because this current appears only for values of the membrane potential greater than -30mV which is in the range where normally this kind of channels become active. Moreover, as we have collected data from traces only after 20ms from the stimulus onset, if Na+ channels are induced, they should be of the non-inactivating type.
Figure 3: The effect of TCDD exposure. Comparison of the total density currents between the two groups (control and treated) for each couple of solution (∗ = p < 0.05).
Figure 4: Differences of the mean density currents between solutions C and D in both treated and control group (a); differences of the mean density currents between solutions B and D in the treated group (b) (∗ = p < 0.05).
The fact that, the current contributed by N a+ is of unexpectedly of the outward type, could be explained hypothesizing that the Na+ current activates a pumping system that pumps out a quantity of current significantly larger than the inward current produced by the ionic channels. Pumps sensitive to local ionic changes of concentrations have been largely described [31] and complex transport systems have been also identified in a cell line similar to ours [32-35]. However, these results are preliminary and, of course, need of further confirmations to be supported.
Conclusion
By the previous results and discussions, we can summarize the following conclusions:
a) Treatment of MDBK cells with TCDD produces a significant increase of the membrane currents obtained at different levels of membrane voltage;
b) The main effect is produced on currents contributed by Cl− ions which flow through the membrane in an ohmic manner;
c) The density of K+ current probably changes after TCDD exposure;
d) TCDD probably activates a Na+ conductance which we have hypothesized to become an outward current thanks to activation of a (newly or pre-existent) pumping system which produces an efflux of ions larger than the influx one.
Acknowledgement
We thank Dr. Vito Di Maio for his fruitful comments on the manuscript.
References
- Ames J, Warner M, Mocarelli P, Brambilla P, Signorini S, et al. (2018) AHR gene-dioxin interactions and birthweight in the Seveso Second Generation Health Study. Int J Epidemiol 47(6): 1992-2004.
- Mandal PK (2005) Dioxin: A review of its environmental effects and its aryl hydrocarbon receptor biology. J Comp Physiol B 175(4): 221-230.
- White SS, Birnbaum LS (2009) An overview of the effects of dioxins and dioxin-like compounds on vertebrates, as documented in human and ecological epidemiology. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev 27(4): 197-211.
- Fiorito F, Santamaria R, Irace C, De Martino L, Iovane G (2017a) 2,3,7,8- tetrachlorodibenzo-p-dioxin and the viral infection. Environ Res 153 :27-34.
- Legare M, Hanneman W, Barhoumi R, Burghardt R, Tiffany- Castiglioni E (2000) 2,3,7,8-Tetrachlorodibenzo-p-dioxin alters hippocampal astroglia-neuronal gap junctional communication. Neurotoxicology 21(6): 1109-1116.
- Nayyar T, Zawia N, Hood D (2002) Transplacental effects of 2,3,7,8- tetrachlorodibenzo-p-dioxin on the temporal modulation of Sp1 DNA binding in the developing cerebral cortex and cerebellum. Exp Toxicol Pathol 53(6): 461-468.
- Jacobson J, Jacobson S (1996) Intellectual impairment in children exposed to polychlorinated biphenyls in utero. N Engl J Med 335(11): 783-789.
- Boverhof D, Tam E, Harney A, Crawford R, Kaminski N, et al. (2004) 2,3,7,8-Tetrachlorodibenzo-p-dioxin induces suppressor of cytokine signaling in murine B cells. Mol Pharmacol 66(6): 1662-1670.
- IARC (1997) IARC monographs on the evaluation of carcinogenic risks to humans. In: Polycholorinated dibenozoparadioxins and polychlorinated dibenzofurnas.
- Kim SY, Yang JH (2005) Neurotoxic effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin in cerebellar granule cells. Exp Mol Med 37(1): 58-64.
- Fernandez M, Paradisi M, D’Intino G, Del Vecchio G, Sivilia S, et al. (2010) A single prenatal exposure to the endocrine disruptor 2,3,7,8- tetrachlorodibenzo-p-dioxin alters developmental myelination and remyelination potential in the rat brain. J Neurochem 115(4): 897-909.
- Sanchez-Martin FJ, Salguero FP M, Merino JM (2010) 2,3,7,8- Tetrachlorodibenzo-p-dioxin induces apoptosis in Neural Growth Factor (NGF)-differentiated pheochromocytoma PC12 cells. Neurotoxicology 31(3): 267-276.
- Hogenesch JB, Chan WK, Jackiw VH, Brown RC, Gu YZ, et al. (1997) Characterization of a subset of the basic-helix-loophelix- PAS superfamily that interacts with components of the dioxin signaling pathway. J Biol Chem 272(13): 8581-8593.
- Mulero-Navarro S, Fernandez-Salguero PM (2016) New trends in aryl hydrocar- bon receptor biology. Front Cell Dev Biol 4: 45.
- Morales-Hernandez A, Sanchez-Martin F, Hortigon-Vinagre M, Henao F, Merino J (2012) 2,3,7,8-Tetrachlorodibenzop- dioxin induces apoptosis by disruption of intracellular calcium homeostasis in human neuronal cell line SHSY5Y. Apoptosis 17(11): 1170-1181.
- Bock K, Kohle C (2006) Ah receptor: Dioxin-mediated toxic responses as hints to deregulated physiologic functions. Biochem Pharmacol 72(4): 393-404.
- Chopra M, Schrenk D (2011) Dioxin toxicity, aryl hydrocarbon receptor signaling, and apoptosis-persistent pollutants affect programmed cell death. Crit Rev Toxicol 41(4): 292-320.
- Mimura J, Fujii-Kuriyama Y (2003) Functional role of AhR in the expression of toxic effects by TCDD. Biochim Biophys Acta 1619(3): 263-268.
- Fiorito F, Cantiello A, Granato G, Marf`e G, Ciarcia R, et al. (2014a) Modulation of telomerase activity, bTERT and c-Myc induced by 2,3,7,8-tetrachlorodibenzo-p-dioxin during Bovine Her- pesvirus 1 infection in MDBK cells. Toxicol In Vitro 28(1): 24-30.
- Fiorito F, De Martino L (2014b) The presence of dioxin in kidney cells induces cell death with autophagy. Autophagy Cancer, Other Pathol Inflammation, Immunity, Infect Aging 1: 145-155.
- Fiorito F, Pagnini U, De Martino L, Montagnaro S, Ciarcia R, et al. (2008a) 2,3,7,8-tetrachlorodibenzo-p- dioxin increases Bovine Herpesvirus Type-1 (BHV-1) replication in Madin- Darby Bovine Kidney (MDBK) cells in vitro. J Cell Biochem 103(1): 221-233.
- Fiorito F, Ciarcia R, Granato G, Marf`e G, Iovane V, et al. (2011) 2,3,7,8-tetrachlorodibenzo-p-dioxin induced au-tophagy in a bovine kidney cell line. Toxicology 290(2-3): 258-270.
- Fiorito F, Marf`e G, De Blasio E, Granato G, Tafani M, et al. (2008b)2,3,7,8-tetrachlorodibenzo-p-dioxinregulates bovine herpesvirus type 1 induced apoptosis by modulating Bcl-2 family members. Apoptosis 13(10): 1243-1252.
- Fiorito F, Irace C, Di Pascale A, Colonna A, Iovane G, et al. (2013) 2,3,7,8-Tetrachlorodibenzo-p-dioxin promotes BHV- 1 infection in mammalian cells by interfering with iron homeostasis regulation. PLoS One 8(3): e58845.
- Fiorito F, Iovane V, Marullo A, Costagliola A, Granato GE, et al. (2017b) 2,3,7,8-Tetrachlorodibenzo-p-dioxin influences bovine herpesvirus 1 replication through upregulation of SIRT3 and cytoskeletal reorganization. Vet Res Commun 41(4): 299-306.
- Xie A WD Walker NJ (2006) Dioxin (TCDD) enhances triggered after depolarizations in rat ventricular myocytes. Cardiovasc Toxicol 6(2): 99-110.
- Levin M (2012) Molecular bioelectricity in developmental biology: New tools and recent discoveries: Control of cell behavior and pattern formation by transmembrane potential gradients. Bioessays 34(3): 205-217.
- Kondratskyi A, Kondratska K, Skryma R, Klionsky D, Prevarskaya N (2018) Ion channels in the regulation of autophagy. Autophagy 14(1): 3-21.
- Kondratskyi A, Kondratska K, Skryma R, Prevarskaya N (2015) Ion channels in the regulation of apoptosis. Biochim Biophys Acta 1848(10 Pt B): 2532-2546.
- Santamaria R, Fiorito F, Irace C, De Martino L, Maffettone C, et al. (2011) 2,3,7,8- Tetrachlorodibenzo-p-dioxin impairs iron homeostasis by modulating iron related proteins expression and increasing the labile iron pool in mammalian cells. Biochim Biophys Acta 1813(5): 704-712.
- Jigyasi J, Kundu R (2013) Effects of low dose dioxin on membrane bound ion dependent ATPases in mice kidney. IOSR J Env Sci Tox Food Tech 2: 43-48.
- Lang F, Paulmichl M (1995) Properties and regulation of ion channels in MDCK cells. Kidney Int 48(4): 1200-1205.
- Rocafull M, Thomas L, del Castillo J (2012) The second sodium pump: from the function to the gene. Pflugers Arch 463(6): 755-777.
- Thomas L, Rocafull M, Del Castillo J (2013) Is the second sodium pump electrogenic? Biomed Res Int 2013: 698674.
- Fiorito F, Marf`e G, Granato GE, Ciarcia R, De Blasio E, et al. (2010) 2,3,7,8- Tetrachlorodibenzo-p-dioxin modifies expression and nuclear/cytosolic localization Of Bovine Herpesvirus 1 Immediate-Early Protein (bICP0) during infection. J Cell Biochem 111(2): 333-342.

 Research Article
Research Article