Abstract
Bacteriophage (phage) treatment has proven successful for the treatment of bacteremia caused by Staphylococcus aureus (SA). Surprisingly, a recent study shows that blood inhibits SA phage propagation in vitro. Here we investigate putative in vitro inhibition of SA and/or phage K propagation by human whole blood, serum and plasma. We studied the ability of phage K to produce progeny in three SA strains growing in different media. The ability of S. aureus to multiply in human whole blood, serum and plasma were determined by CFU (Colony Forming Units) titration. Phage K propagation was evaluated by PFU (Plaque Forming Units) titration using on the double agar overlay technique. S. aureus grows robustly in whole blood and serum. By contrast, whole blood, serum and plasma strongly inhibit the propagation of phage K in three different SA strains. Our results demonstrate that SA-phage propagation inhibition by blood is not due to lack of growth of the host, and suggest an effect of the liquid components of human blood preventing the propagation of phage K.
Keywords: Propagation of S. aureus Phage K in Presence of Human Blood
Introduction
The specter of antimicrobial resistance has been present as far back as antibiotic treatment for bacterial infections has been available [1]. The current state of bacterial resistance to antibiotics coupled with inadequate development of more potent drugs is increasing the proportion of untreatable bacterial infections [2,3]. Currently more than 23,000 deaths are attributed to multi-drug resistant microorganisms in the United States each year, with worldwide deaths from resistance estimated to reach 10 million by 2050 [4]. One such resistant bacteria, Methicillin Resistant Staphylococcus aureus (MRSA), is of special international concern [2]. MRSA represents a primary cause of hospital acquired infections and is responsible for over 12,000 related deaths in the United States each year [5]. Identifying efficacious novel therapies to combat the inexorable increase in MRSA commensurate is imperative to public health. Among the novel approaches to combat bacterial resistance, phages have been utilized as a standalone and adjunct of antibiotic treatment to combat antibiotic resistance [6]. Phages are prokaryotic viruses that infect and replicate within bacteria. In the case of lytic phages, the result of this infection is bacterial lysis/killing and release of phage progeny in other sites of infection in the body which continues the life cycle. Phage therapy employs targeted application of lytic phage(s) to infect and kill a specific pathogenic bacterium [7-10].
Phage therapy is highly relevant for military medicine, both on the battlefield and in military treatment facilities in the United States. Phage therapy during World War II lead to significant decreases in gangrene and amputations.10 During recent conflicts almost 9% of all United States combat deaths were caused by wound exposure to bacteria in the battlefield; these infections have a high prevalence of multidrug-resistance.10 Western medicine has reembraced phage therapy due to the rapid increase in bacterial multidrug resistance at the end of the 20th century [7,9, 11-13]. Phage therapy can clear Enterococcus and Vibrio infections in mouse and rabbit models and protect against S. aureus (SA) and E. coli infections in mouse model studies [9,13-18]. Moreover, the efficacy and safety of phage therapy has also been demonstrated in humans [19-23].In the case of MRSA-mediated septicemia, therapy with phage S13′ has proven effective in mice [24]. These results are important given over half a million cases of sepsis occur each year in the United States, resulting in up to 50 percent mortality despite antibiotic treatment [25].
Interestingly, a recent study has shown that SA phage SATA8505A is effective on the treatment of MRSA skin infections in mice, but the phage is unable to propagate and kill MRSA ex-vivo in the presence of human whole blood [26]. This raises the question on how phages could be effective in the treatment of SA bacteremia since they can’t kill the host in the blood matrix. The authors suggest that a possible reason for this is a lack of SA growth in blood. Since phages required active growth of their host for their own multiplication, this will explain the results. Due to the paucity of data regarding SA killing in blood by phages, or even sound body of information on the ability of this bacteria to growth in blood, additional studies on this subject are important to further evaluate phage therapy against MRSA infections. Here we evaluate the effect of human blood on phage activity against SA in vitro. We focused on SA- phage K due to its broad range and strong virulence toward SA, and on phage K propagation using three clinical SA strains.
Materials and Methods
Bacterial Strains, Phage K, and Growth Methods
S. aureus strains ATCC 11987 (SA11987), 1415 (SA1415) and 1012 (SA1012) selected from our bacterial collection. SA11987 is a common with widespread research use [27]. SA1415 and SA1012 are respectively MSSA and MRSA nosocomial clinical isolates from Fort Benning, GA [28]. Phage K was purchased from ATCC (19685- B1) [29]. Bacteria and phage K were commonly propagated in tryptic soy broth (TSB; Thermo Fisher Scientific, Waltham, MA) at 37°C.
Blood Source
Sodium fluoride anticoagulated Group AB RhD positive human whole blood, pooled frozen off-clot Group AB RhD positive serum from male donors and frozen group O RhD positive plasma from male donors were utilized. These products were sourced from paid donors by Zenbio Incorporated (Durham, NC) and shipped within 2 business days of collection. Group AB RhD positive whole blood and serum were selected to minimize naturally occurring ABO blood group antibodies present. Sodium fluoride anticoagulant was selected to minimize available amino acid and sugar sources for SA which could confound growth results. Group O RhD positive frozen plasma was selected an alternative blood group component in the event phage K was unable to propagate in whole blood and serum.
Double Agar Layer Technique
Phage K titers (Plaque Forming Units/ml or pfu/ml) were determined by visualization of plaques on TSA plates using the soft agar overlay technique previously described [21]. Briefly, 100µl aliquot of each sample was mixed with 900µl of phosphate buffer saline pH 7.4 (PBS) and serially diluted as appropriated. 10µl of each dilution was then used to infect 100µl of exponential growth S. aureus, incubated at 37°C for 5 minutes, added to 2.5ml of melted top agar (59°C) and poured immediately on a TSA plates. All plates were incubated for 24 hours at 37°C and the phage plaques enumerated.
Titration of SA and Phage K
The number of SA cells in liquid cultures were estimated by determining the number of Colony Forming Units (CFU) as previously described [18]. Briefly, the cultures were sequentially diluted in PBS followed by plating in TSB-agar plates and incubated overnight at 37°C. Colonies were counted and this number used to extrapolate the original cell concentration. The infection of SA with phages were done at Multiplicity of Infection (MOI) of 10, to avoid killing form without, unless indicated otherwise [30]. The number of phage K particles in liquid cultures were estimated by determining the number of Plaque Forming Units (PFU) as previously described [18]. The cultures were sequentially diluted in PBS followed by plating in TSB-agar plates and incubated overnight at 37°C. Plaques were counted and this number used to extrapolate the original phage K concentration.
Results
S. aureus Grows in Blood in vitro
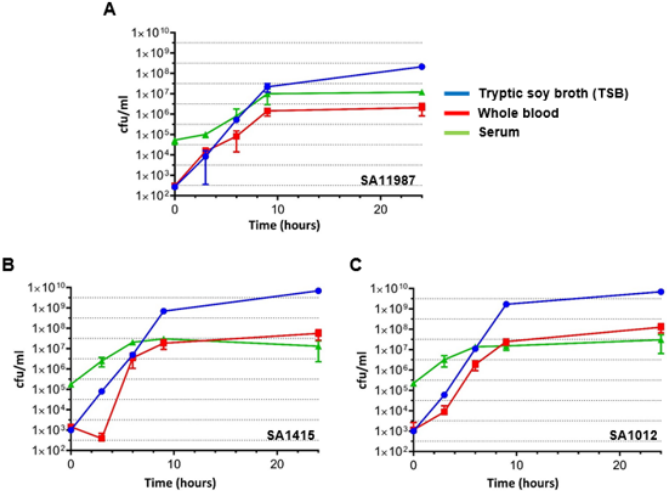
In order to evaluate the putative inhibitory effect of human blood on the growth of SA in vitro, we characterized the growth of three SA strains directly in undiluted human blood. SA11987, SA1415 (MSSA) and SA1012 (MRSA) overnight cultures were diluted 1/100 in fresh TSB and allowed the cultures to proceed to OD600 of 0.1 in order to begin with cells in exponential growth phase. At this point, the cultures were further diluted to OD600 of 0.001 (~106 CFU/ml) in PBS [18]. 20µl of this dilution was inoculated in 1ml of media (either TSB or the different blood media, as indicated) and incubated at 37°C with constant agitation (200 rpm). Samples were taken at 0, 3, 6, 9- and 24-hour time points, and the CFU/ml calculated. As can be seen in Figure 1A, SA11987 demonstrated increases in CFU/ml ~200,000 fold after 9 hours incubation in TSB. This is equivalent to 17.6 generations (log2n =log2x105 ), 31 minutes generation time (g=t/n). SA11987 growth in blood is not halted and shows a robust growth, although slower than in TSB (in 9 hours, ~10,000-fold increase; 13.3 generations; 40 minutes duplication time). Similar results were obtained for SA1415 and SA1012 (Figure 1B & 1C). Therefore, although whole blood is not as good growth media as TSB, whole blood supports a robust in vitro growth of S. aureus. The growth of all S. aureus strains in serum demonstrated nearly identical growth rates across the strains, with growth observed from initial concentrations of 105 CFU/ml to 107 CFU/ml at 9 hours incubation. As in whole blood, the growth of all S. aureus strains was within ~1 log of their growth in TSB control medium after 24 hours.
Figure 1: S. aureus Multiplies in Human Whole Blood and Serum in vitro
a. Growth of S. aureus 11987 incubated in various media in vitro at 37°C. The blue, red and green lines correspond to the growth plots of the bacteria in TSB, whole blood and serum respectively.
b. Growth of S. aureus 1415 incubated in various media in vitro at 37°C. The color coding of the growth plots as in panel A.
c. Growth of S. aureus 1012 incubated in various media in vitro at 37°C. The color coding of the growth plots as in panel A. Cfu, Colony Forming Units.
Phage K Propagation in S. aureus is Inhibited in Presence of Human Blood
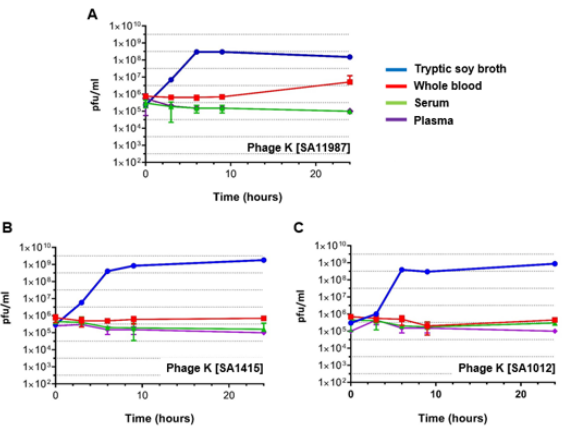
We followed a similar approach for the evaluation of the effect of blood on the propagation of phages. Exponential SA cultures were infected with phage K at time 0, samples were taken at 0, 3, 6, 9 and 24 hour time points, and the phage titers were determined using the double layer agar technique. The regular propagation curve of phage K infected into S. aureus inoculums in TSB medium at a MOI of 10 in Figure 2 demonstrates the replication of phage K, with titers increasing ~1,000 fold after 3 hours for all S. aureus strains. By contrast the titer of phage K did not significantly increase during this period of time when SA11987, SA1415 or SA1012 were cultured in whole blood (Figure 2). Thus, blood inhibits phage K propagation and this effect is not due to the lack of growth of its host. To clarify if phage K inhibition is mediated by the cellular components of blood, or by constituents of the coagulation cascade (fibrinogen and procoagulant factors), we repeated the experiments in using plasma and serum as culture media respectively. Our results suggest neither of the aforementioned factors are integral to inhibition of phage K propagation (Figure 2).
Figure 2: Phage K Fails to Kill S. aureus in Human Whole Blood and Serum in vitro
a. Propagation of phage K in S. aureus 11987 incubated in various media in vitro at 37°C. The blue, red, green and purple lines correspond to the phage propagation plots in TSB, whole blood, serum and plasma respectively.
b. Propagation of phage K in S. aureus 1415 incubated in various media in vitro at 37°C. The color coding of the propagation plots as in panel A.
c. Propagation of phage K in S. aureus 1012 incubated in various media in vitro at 37°C. The color coding of the propagation plots as in panel A. Pfu, Plaque Forming Units.
Conclusion
Due to the obvious implications for the future use of phage therapy in S. aureus bacteremia cases, we investigated the recently described inhibition of SA phage propagation by blood in vitro 26. This phenomenon could be ascribed simply to the lack of growth of S. aureus in blood, thus indirectly depriving the phages of the bacterial metabolic resources necessary for the completion of their life cycle 26. However, we show here that S. aureus does multiply in human whole blood and serum. Nevertheless, we also show that despite the growth of S. aureus in blood, phage K propagation is compromised. Therefore, our results support the inhibition of SA phages by blood in vitro and rule out that this effect is due to lack of growth of the bacterial host.
Acknowledgment
The authors would like to acknowledge the contributions of LCDR Robert Hontz and Kimberly Bishop- Lilly for their leadership and vision in the planning of this study. The authors are service member or federal/contracted employee of the United States government. This work was prepared as part of my official duties. Title 17 U.S.C. 105 provides that `copyright protection under this title is not available for any work of the United States Government.’ Title 17 U.S.C. 101 defines a U.S. Government work as work prepared by a military service member or employee of the U.S. Government as part of that person’s official duties. The views expressed in this article reflect the results of research conducted by the author and do not necessarily reflect the official policy or position of the Department of the Navy, Department of Defense, nor the United States Government.
References
- Fleming A (1945) Nobel lecture. Physiology or Medicine.
- Martens E, Demain AL (2017) The antibiotic resistance crisis, with a focus on the United States. The Journal of Antibiotics 70: 520-526.
- (2014) World Health Organization. Antimicrobial resistance: global report on surveillance, Geneva.
- ONeill J (2016) Review on antimicrobial resistance: tackling a crisis for the health and wealth of nations. HM Government, London, England.
- Debarbieux L, Pimay JP, Berbeken G, De Vos D, Merabishvili M, et al. (2016) A bacteriophage journey at the European Medicines Agency. Microbiology Letters 363(2).
- (2015) National Action Plan for Combating Antibiotic-Resistant Bacteria. The White house, Washington.
- Abedon ST, Kuhl SJ, Blasdel BG, Elizabeth Martin Kutter (2011) Phage treatment of human infections. Bacteriophage 1(2): 66-85.
- Reindel R, Fiore CR (2017) Phage therapy: considerations and challenges for development. Clinical Infectious Diseases 64(11): 1589-1590.
- Sulakvelidze A, Alavidze Z, Morris JG (2001) Bacteriophage therapy. Antimicrobial agents and chemotherapy 45(3): 649-659.
- Gelman D, Eisenkraft D, Chanishvili N, Nachman D, Coppenhagem Glazer S, et al. 2018. The history and promising future of phage therapy in the military service. Journal of Trauma and Acute Care Surgery 85(1S Suppl 1): S18-S26.
- Young R, Gill JJ (2015) Phage therapy redux-What is to be done?. Science 350(6265): 1163-1164.
- Matsuzaki S, Rashel M, Uchiyama J, Shingo Sakurai, Takako Ujihara, et al. (2005) Bacteriophage therapy: a revitalized therapy against bacterial infectious diseases. Journal of infection and chemotherapy 11(5): 211- 219.
- Biswas B, Adhya S, Washart P, Brian Paul, Andrei N Trostel, et al. (2011) Bacteriophage Therapy Rescues Mice Bacteremic from a Clinical Isolate of Vancomycin-Resistant Enterococcus faecium. Infection and Immunity 70(1): 204-210.
- Cerveny KE, DePaola A, Duckworth DH, Gulig PA (2011) Phage therapy of local and systemic disease caused by Vibrio vulnificus in iron-dextrantreated mice. Infection and immunity 70(11): 6251-6262.
- Soothill JS (1992) Treatment of experimental infections of mice with bacteriophages. Journal of medical microbiology 37(4): 258-261.
- Smith HW, Huggins MB (1982) Successful treatment of experimental Escherichia coli infections in mice using phage: its general superiority over antibiotics. Microbiology 128(2): 307-318.
- Hraiech S, Bregeon F, Rolain JM (2015) Bacteriophage-based Therapy in Cystic Fibrosis-associated Pseudomonas aeruginosa Infections: rationale and current status. Drug Design Development and Therapy 9: 3653.
- Estrella LA, Quinones J, Henry M, Ryan M Hannah, Robert K Pope, et al. (2016) Characterization of novel Staphylococcus aureus lytic phage and defining their combinatorial virulence using the OmniLog® system. Bacteriophage 6(3): e1219440.
- Bruttin A, Brüssow H (2005) Human volunteers receiving Escherichia coli phage T4 orally: a safety test of phage therapy. Antimicrobial agents and chemotherapy 49(7): 2874-2878.
- Marza JS, Soothil JS, Boydell P, Collyns TA (2006) Multiplication of therapeutically administered bacteriophages in Pseudomonas aeruginosa infected patients. Burns 32(5): 644-646.
- Schooley RT, Biswas B, Gill JJ, Hernandez Morales A, Lancaster J, et al. (2017) Development and use of personalized bacteriophagebased therapeutic cocktails to treat a patient with a disseminated resistant Acinetobacter baumannii infection. Antimicrobial Agents and Chemotherapy 61(10): e00954 -17.
- Rhoads DD, Wolcott RD, Kuskowski MA, BM Wolcott, LS Ward, et al. (2009) Bacteriophage therapy of venous leg ulcers in humans: results of a phase I safety trial. Journal of Wound Care 18: 237-243.
- Wright A, Hawkins AH, Anggard EE, , Harper DR (2009) A controlled clinical trial of a therapeutic bacteriophage preparation in chronic otitis due to antibiotic-resistant Pseudomonas aeruginosa; a preliminary report of efficacy. Clinical Otolaryngology 34(4): 349-357.
- Takemura Uchiyama I, Uchiyama J, Osanai M, Morimoto N, Asagiri T, et al. (2014) Experimental phage therapy Experimental phage therapy against lethal lung-derived septicemia caused by Staphylococcus aureus in mice. Microbes and Infection 16(6): 512–517.
- Matthay MA (2001) Severe sepsis-a new treatment with both anticoagulant and anti-inflammatory properties. Massachusetts Medical Society 344(10): 759-762.
- Pincus NB, Reckhow JD, Saleem D, Jammeh ML, Datta SK, et al. (2014) Strain Specific Phage Treatment for Staphylococcus aureus Infection Is Influenced by Host Immunity and Site of Infection. PLoS One 10(4): e0124280.
- Ralston DJ, Krueger AP (1954) The Isolation of a Staphylococcal phage variant susceptible to an unusual host control. The Journal of General Physiology 37(5): 685-716.
- Wisplinghoff H, Bischoff T, Tallent SM, Seifert H, Wenzel RP, et al. (2004) Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clinical Infectious Disease 39(3): 309-317.
- OFlaherty S, Ross RP, Meaney W, Fitzgerald GF, Elbreki MF, et al. (2005) Potential of the polyvalent anti-Staphylococcus bacteriophage K for control of antibiotic-resistant staphylococci from hospitals. Applied and environmental microbiology 71(4): 1836-1842.
- Abedon ST (2011) Lysis from Without. Bacteriophage 1(1): 46-49.

 Short Communication
Short Communication