Abstract
Cell blocks are a valuable, widely demonstrated tool for obtaining paraffin-embedded cell sections on which to perform standard staining procedures and, when appropriate, immunohistochemistry or other ancillary tests. We describe an innovative application of a fully automated inclusion and embedding procedure, routinely used for small biopsies, to create cell blocks thereby simplifying methodology and improving quality for cytological diagnosis.
Abbreviations: CBs: Cell Blocks; RCF: Relative Centrifugal Field; TCB: Traditional Cell Blocks; IHC: Immunohistochemical
Introduction
Minimally invasive approaches have become standard operating procedure in the collection of tissue and cells for diagnosis, prognosis and therapeutic prediction. The pathologist’s ability to obtain answers from cytologic samples, as well as from small tissue biopsies, has become ever more crucial. Cytology is considered an accurate test for evaluation of various superficial and deep seated lesions, such as thyroid nodules [1,2], abdominal masses [3] and breast lesions [4], as well as cavity free fluids (such as pleural liquid and ascites) with general high sensitivity and specificity when in expert hands. Cytology is cost effective, has low complication rates and permits optimal patient management. In the last years many laboratories have introduced cell blocks (CBs) in their routine cytopathology work as an important diagnostic aid providing additional morphological detail and ensuring adequate immunohistochemistry and molecular tests [5]. Although various methods have been proposed, they all suffer from advantages and disadvantages in terms of increase of work times, complexity of procedures, costs and adequacy of cellularity. We propose a new automated method which increases cellular yield and quality of biological fluids while minimizing laboratory times and costs. Following the introduction to our laboratory of a fully automated processor which also embeds in paraffin (LOGOS, Milestone Medical, Bergamo, Italy) used for small biopsies, we applied the same method to include cytologic samples, which can then be treated exactly as traditional cell blocks.
Materials and Methods
A fully automated tissue processor with microwave hybrid processing technology and automated embedding (LOGOS, Milestone Medical, Bergamo, Italy [6]) was tested in our laboratory for fast track small biopsy samples. We have an active Quality Assurance team which checks all new procedures introduced in the laboratory and tests new and innovative possibilities for the translation of results to other areas of practice. Therefore, as a by lane of our automated processing/embedding project, we extended this procedure, tweaking and modifying protocols for cytological samples.
Cytological Procedure – Automated Cell Blocks (ACB)
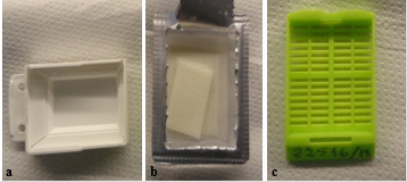
While processing/embedding followed the usual standard fast track protocol for small tissue samples (2h 24 minutes protocol - see Table 1), an initial step was added for cyto-samples. Six cases with abundant, left over liquids from routine diagnostic cases were selected: one pleural and 5 peritoneal fluids - all cases were anonymized. Fresh liquids were centrifuged at 1,000 relative centrifugal field (RCF) for 10 minutes, and the sediments were directly included without need of either formalin or methanol pretreatment. Once centrifuged, the samples were placed in dedicated kits (Synergy Kit, Milestone Medical, Bergamo, Italy) composed of plastic molds and pads (made from biomaterial which can be processed, embedded and microtome cut) which were placed on top of the molds to entrap cells and avoid dispersion (Figure 1). All ACBS were subsequently covered with plastic bio-cassettes and processed/embedded using the LOGOS dedicated processor.
Figure 1: Synergy kit (Milestone Medical, Bergamo, Italy) used for processing embedding:
a) Mold
b) Biomaterial sponge
c) Plastic biocassette
Cytological Procedure - Traditional Cell Blocks (TCB)
For comparison, traditional cell blocks (TCB) from the same liquids were obtained by routine non-automatic cyto-inclusion technique using our standard laboratory processor. In particular, after centrifuging, the sediment is placed between two sponges, fixed in alcohol and routinely processed for a standard 16 hours. Embedding is manually performed by scraping the surface of the sponges and collecting all visible material.
Evaluation of Samples
All cell blocks, both ACB and TCB, were stained with haematoxylin and eosin and evaluated for cellularity, cell cohesion, nuclear chromatin pattern, nuclear membrane irregularities and the presence of nucleoli. Additional immunohistochemical (IHC) studies were performed on one case containing cancer cells for further cell immunophenotyping. Appropriate on-slide positive immunohistochemical controls were performed to ensure accurate interpretation of results [7]. A comparison of turnaround times between ACB and TCB was also assessed.
Results
Adequacy of samples was obtained with both ACB and TCB, however a higher number of cells was present with the ACB technique. Cellularity, presence of nucleoli, nuclear membrane detail and architectural patterns were comparable across both methods (Figure 2a). Immunohistochemistry was also adequate and assessable in ACB (Figure 2b). Main differences between the two methods consisted in a reduction of turnaround times for the ACB method. Indeed, while the cell block preparation times were similar up to processing, this step of the protocol was drastically shorter for ACB (2 hours and 24 minutes) versus TCB (16 hours). Furthermore, all embedding related work times are not present for the ACB technique.
Figure 2: a) Haematoxylin and eosin section of ACB sample showing optimal cell details: the pink amorphous material surrounding the cells is the biomaterial sponge used in the Synergy Kit (magnification x20).
b) Immunoreactivity for CEA in an ACB samples of adenocarcinoma cells in peritoneal fluid (magnification x60).
Conclusion
The use of cell blocks in diagnostic cytology is an important tool for optimizing cell evaluation on multiple sections as well as making cells available for further techniques. A variety of cell block preparation methods exist [8] from agarose embedding to entrapment of cells in dedicated filters (which can be treated as tissue samples). Among these techniques, the traditional thromboplastin method demonstrates superiority in terms of ease of preparation and optimal cellularity, but it requires overnight formalin fixation and subsequent manual embedding, resulting in longer case turnaround times (at least 24 hours in), higher operating costs and comprehensive increase of laboratory work load [5]. A new automated method of producing cell blocks, the Client TM Automated Cell Block System (Hologic Corporation, Marlborough, MA, USA) has been proposed [9-10], however a main disadvantage of this system the fact that it is based on methanol fixation instead of the routinely used formalin-based fixation, which may be problematic for immunohistochemistry testing. Other automated rapid processing techniques are available which permit processing times of about 3 hours [11], however none of these follows with automated embedding. The proposed ACB method is an additional fully automated cell block method which may be used with success in any pathology laboratory equipped with LOGOS. Furthermore, this automated technique optimizes cell block preparation, reducing processing times and standardizing embedding procedures while preserving high cellularity and validity of ancillary testing.
Competing Interests
MC received BJSTR publication fee from A. Menarini diagnostics.
References
- Chiofalo MG, Signoriello S, Fulciniti F, Avenia N, Ristagno S, et al. (2018) Predictivity of clinical, laboratory and imaging findings in diagnostic definition of palpable thyroid nodules. A multicenter prospective study. Endocrine 61(1): 43-50.
- Saleh HA, Hammoud J, Zakaria R, Khan AZ (2008) Comparison of ThinPrep and cell block preparation for the evaluation of Thyroid epithelial lesions on fine needle aspiration biopsy. Cytojournal 5: 3.
- Stewart CJ, Coldewey J, Stewart IS (2002) Comparison of fine needle aspiration cytology and needle core biopsy in the diagnosis of radiologically detected abdominal lesions. J Clin Pathol 55(2): 93-97.
- Moschetta M, Telegrafo M, Carluccio DA, Jablonska JP, Rella L, et al. (2014) Comparison between fine needle aspiration cytology (FNAC) and core needle biopsy (CNB) in the diagnosis of breast lesions. G Chir 35(7-8): 171-176.
- Nambirajan A, Jain D (2018) Cell blocks in cytopathology: An update. Cytopathology 29(6): 505-524.
- https://www.milestonemedsrl.com/product/logos/
- Bragoni A, Gambella A, Pigozzi S, Grigolini M, Fiocca R, et al. (2017) Quality control in diagnostic immunohistochemistry: integrated onslide positive controls. Histochem Cell Biol 148(5): 569-573.
- Krogerus L, Kholová I (2018) Cell Block in Cytological Diagnostics: Review of Preparatory Techniques. Acta Cytologica 62(4): 237-243.
- Prendeville S, Brosnan T, Browne TJ, Mc Carthy J (2014) Automated Cellient(™) cytoblocks: better, stronger, faster? Cytopathology 25(6): 372-380.
- Wagner DG, Russell DK, Benson JM, Schneider AE, Hoda RS, et al. (2011) Cellient™ automated cell block versus traditional cell block preparation: a comparison of morphologic features and immunohistochemical staining. Diagn Cytopathol 39(10): 730-736.
- Zhao J, Lin DL, Zhai LH, Wang JG (2014) Evaluation of ultrasoundprocessed rapid cell blocks in the cytopathologic diagnosis of cavity fluids. Acta Cytol 58: 182-191.

 Short Communication
Short Communication