Abstract
The determination of the expression of epidermal growth factor receptor 2 gene (HER2) by immunohistochemistry is very important to determine the final decision treatment of breast cancer because there are a correlation between HER2 expression and response to anti HER2 therapy . There is the possibility of finding a equivocal expression of HER2 that requires a more accurate determination. We analyse the incidence of equivocal determination of HER2 and their correlation with clinicopathological variables.
Keywords: Epidermal Growth Factor Receptor 2 Gene; Breast Cancer; In Situ Hybridization Techniques
Introduction
Epidermal growth factor receptor 2 gene (HER2) is overexpressed in approximately 18% to 20% of primary breast cancers [1] providing a special prognostic and predictive factor. Since 2001, the use of trastuzumab, the first antiHER2 therapy, has shown a significant survival improvement when combined with chemotherapy in the metastatic setting [2] other pivotal trials have confirmed this benefit in early and locally-advanced breast cancer [3-5]. In the last few years new HER-2-targeted drugs such as lapatinib [6] pertuzumab [7] and ado-trastuzumab emtansine (TDM1) [8] have been approved for the treatment of HER2 positive breast cancer. Thus, HER2 status of the patients has been integrated in the clinical practice to guide therapy decisions.
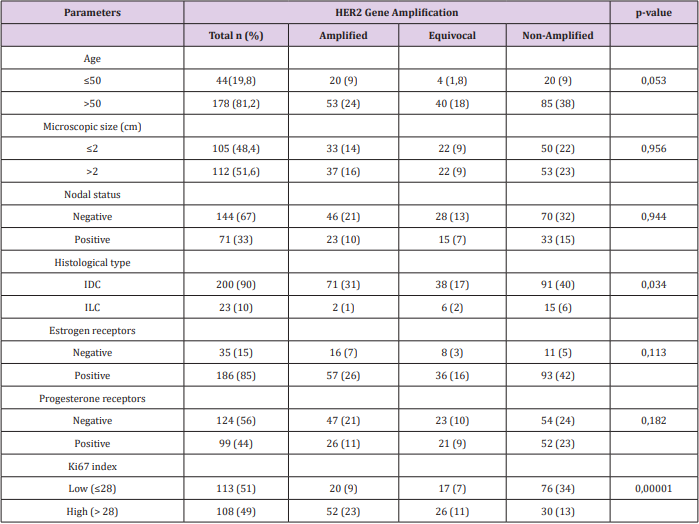
The most widely used guidelines for the diagnosis of HER2 protein overexpression by immunohistochemistry (IHC) are those recommended by the American Society of Clinical Oncology and College of American Pathologists (ASCO/CAP) [9] which categorize the tumor Her2 status: IHC score 0/1+ (IHC-negative), 2+ (IHC equivocal), and 3+ (IHC positive). The IHC-equivocal or borderline 2+ subgroup have ambiguous tumor nature, with a weak HER2 oncoprotein expression. It’s highly crucial for clinicians to determine if these patients are eligible for antiHER2 therapy, so further molecular diagnostic tools are needed to define the Her2 status. In situ hybridization techniques (ISH) are the standard cytogenetic methodology to identify increased number of gene loci (using CEP17 DNA sequence as reference) for accurate HER2 assessment [10]. We analysed a series of 223 patients with IHQ HER2 2+, in which further ISH was performed and correlated with clinicopathological variables that predicting ISH amplification. Main characteristics and its correlation with final ISH results are shown in Table 1. Most of the patients were ER positive (79,8%) and had a high (>20%) proliferative index Ki67 (70%).
Findings
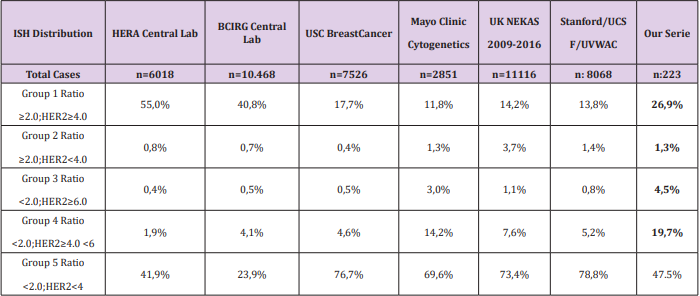
As only patients with HER2 gene amplification have clinical benefit to anti HER2 therapy [11] it is extremely important to define Her2 status in these cases. The ISH techniques show that only 24% of the IHC 2+ tumors have gene amplification [12]. Provided that ISH techniques are complex, we should identify more accurately which cases must be tested by ISH. Many studies have reported that HER-2 overexpression (IHC 3+) or HER-2 gene amplification is associated with high cell differentiation grade, absence of ER or PR expression, DNA aneuploidy, and high Ki-67 [13]. When analyzing factors which could predict HER2 amplification, ER and PR positivity are shown to be related with negative HER-2 status and better prognosis [14]. The most used ASCO guidelines (ASCO/ CAP2013) defines HER2 positivity as: HER2 signal ≥6.0 or ISH ratio >2.0, but the multiple combination possibilities of these two parameters can be categorized in five groups [15].
The results of our cohort of patients are shown in Table 2. The group with HER2 signal ≥4.0 but <6 and ratio <2 is the most controversial and it is defined as equivocal, requiring a new assessment of HER2. In our series, almost 20% of cases are equivocal, considered as a high rate; but still in range of most of the studies [11]. In our cases any variable was found with a predictive value for equivocal ISH result. We found a total of 73 (32%) cases stablished as positive by ISH, which is consistent with other series [11]. A few studies[14] have described the proliferative index Ki67 as the most predictable variable to determine a positive result, with a Odds Ratio (OR) around 3. We performed a logistic regression model to reveal risk factors for HER-2 amplification. The association between clinicopathological variables and HER-2 amplification is shown in Table 1. Cases with high Ki67 had a significantly higher HER-2 amplification than those with low Ki67 (OR =4,318; 95% CI, 2,339-7.971; P<0.000). Unlike other studies, we do not find any correlation between hormonal receptors and ISH status; in contrast with other studies where this correlation is weak compared to that of Ki67. We have generated a risk score that combines the expression of ER and Ki67 with the specific threshold of Her2 positivity. We have found that patients with ER>250 and Ki67<28% show a 19% possibilities to be ISH positive, in contrast to 46% in patients with ER<250 and Ki67>28 (χ2 =23,31, P<0.000).
Conclusion
Accuracy of Her2 status determination in breast cancer is a key point in defining a treatment strategy. However, there are about 20% of cases with equivocal IHC results that require additional techniques such as ISH. A high proportion of these equivocal cases (up to 20%) will require predictive biomarkers. We have found that Ki67 expression correlates to a higher Her2 positivity by ISH, more so if we correlate it with ER expression.
References
- Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, et al. (1987) Human breast cancer: Correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 235: 177-182.
- Slamon DJ, Leyland Jones B, Shak S, Hank Fuchs, Virginia Paton, et al. (2001) Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 344: 783-791.
- Smith I, Procter M, Gelber RD (2007) 2-year follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer: A randomized controlled trial. Lancet 369: 29-36.
- Perez EA, Romond EH, Suman VJ, Jeong JH, Davidson NE, et al. (2011) Four-year follow-up of trastuzumab plus adjuvant chemotherapy for operable human epidermal growth factor receptor 2- positive breast cancer: Joint analysis of data from NCCTG N9831 and NSABP B-31. J Clin Oncol 29(25): 3366-3373.
- Gianni L, Dafni U, Gelber RD, Azambuja E, Muehlbauer S, et al. (2011) Treatment with trastuzumab for 1 year after adjuvant chemotherapy in patients with HER2-positive early breast cancer: A 4-year follow-up of a randomized controlled trial. Lancet Oncol 12(3): 236-244.
- Geyer CE, Forster J, Lindquist D, Stephen Chan, C Gilles Romieu, et al. (2006) Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med 355: 2733-2743.
- Baselga J, Cortes J, Kim SB, Seock Ah Im, Roberto Hegg, et al. (2012) Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N Engl J Med 366: 109-119.
- Verma S, Miles D, Gianni L, Ian E Krop, Manfred Welslau, et al. (2012) Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med 367: 1783-1791.
- Wolff AC, Hammond ME, Hicks DG, Dowsett M, Mc Shane LM, et al. (2013) Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College ofAmerican Pathologists clinical practice guideline update. J Clin Oncol 31(31): 3997-4013.
- Mass RD, Sanders C, Charlene K (2000) The concordance between the clinical trials assay (CTA) and fluorescence in situ hybridization (ISH) in the Herceptin pivotal trials. Proc Am Soc Clin Oncol 19: 75a.
- Afzal M, Amir M, Hassan MJ, Hussain MS, Aziz MN, et al. (2016) Clinical role of HER2 gene amplification and chromosome 17: a study on 154 IHC-equivocal cases of invasive breast carcinoma patients. Tumour Biol 37(7): 8665-8672.
- Singer CF, Tan YY, Fitzal F, Steger GG, Egle D, et al. (2017) Pathological Complete Response to Neoadjuvant Trastuzumab Is Dependent on HER2/CEP17 Ratio in HER2-Amplified Early Breast Cancer. Clin Cancer Res 23(14): 3676-3683.
- Liu C, Zhang H, Shuang C, Lu Y, Jin F, et al. (2010) Alterations of ER, PR, HER-2/neu, and P53 protein expression in ductal breast carcinomas and clinical implications. Med Oncol 27(3): 747-752.
- Ji Y, Sheng L, Du X, Qiu G, Chen B, et al. (2014) Clinicopathological variables predicting HER-2 gene status in immunohistochemistryequivocal (2+) invasive breast cancer. J Thorac Dis 6(7): 896-904.
- Shah MV, Wiktor AE, Meyer RG, Tenner KS, Ballman KV, et al. (2016) Change in Pattern of HER2 Fluorescent In situ Hybridization (ISH) Results in Breast Cancers Submitted for ISH Testing: Experience of a Reference Laboratory Using US Food and Drug Administration Criteria and American Society of Clinical Oncology and College of American Pathologists Guidelines. J Clin Oncol 34(29): 3502-3510.

 Mini Review
Mini Review