Introduction
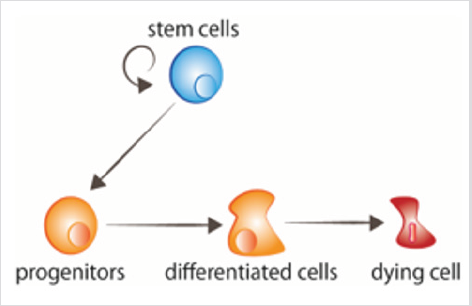
Cell or tissue renewal and regeneration are the two main developmental requirements of adult organisms. Both processes have as starting point a population of stem cells, normally located in a specific environment called the “niche” [1], which provides them the required signals to maintain the stemness properties, or to differentiate to the required different cell types (Figure 1). Stem cell proliferation and differentiation must be coordinated with the death of the cells that need to be replaced. In addition, processes such as cell migration, epigenetics, and cellular communication, are also necessary for proper cell renewal [2,3]. Fast renewal tissues can be recognized by a higher mitotic activity. Conversely, slow renewal tissues contain less mitosis, and may not be easily recognized from non-renewing areas which may also present some mitosis [2]. The fate decisions of stem cells during proliferation directly influence tissue renewal and homeostasis. Therefore, understanding the regulatory mechanisms that sustain a balanced cell division and differentiation is critical. Extracellular signals (e.g., tissue microenvironment, intracellular ROS, and cytokines) as well as intracellular factors (e.g., epigenetic machineries, transcription factors and DNA damage response) are responsible for the regulation of stem cell division.
figure 1: Cell turnover Stem cells proliferate, giving rise to progenitors that thereafter receive the signals to differentiate. Aged cells receive signals to die.
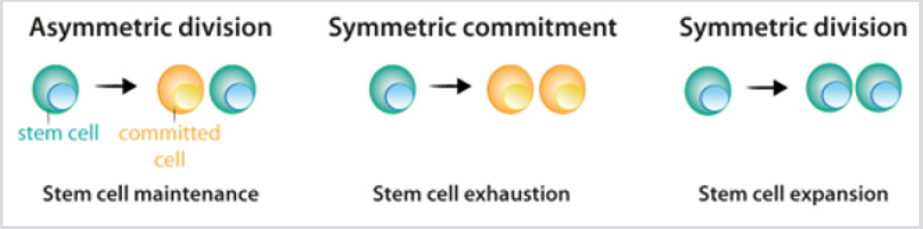
Stem cells show three possible options of division: [1] asymmetric division, in which one stem cell and one committed daughter cell are originated; [2] symmetric commitment, which yields two committed daughter cells; and [3] symmetric division, which yields two daughter cells that maintain stem cell properties [4] Figure 2. Although it could be predicted that asymmetric division is the only mechanism that enables the maintenance of a stable population of stem cells, the current data from lineagetracing experiments demonstrated that in most tissues, the balance between stem cell proliferation and the generation of differentiated offspring is achieved at the level of the whole stem cell population. The loss of stem cells due to differentiation or cell damage, induces symmetric division to fill this gap [5]. After stem cell division, the cells that follow the differentiation process pass through different stages that are defined by a combination of transcription factors that control the activity of the appropriate repertoire of genes, and allow their commitment and terminal differentiation. For each cell lineage the end product of the sequence of decisions is a specific differentiated cell type [6] Figure 3a. Under most circumstances, cellular identity - the product of normal differentiation - is stable within tissues, and its maintenance is crucial for normal tissue function. Such stability is achieved through epigenetic regulation - e.g. histone demethylation and acetylation - that results in heritable patterns of tissue-specific gene expression [3,7].
figure 2: Division pattern of stem cells.
a) During asymmetric division, stem cells give rise to one stem cell, which maintains the stem cell population, and one cell that becomes committed to differentiation.
b) During symmetric commitment, stem cell division gives rise to two daughter cells that became committed to differentiation.
c) During symmetric division, stem cell division gives rise to two stem cells. As explained in the text, current experimental data indicates that the three modes of division can occur while maintaining the stem cell population.
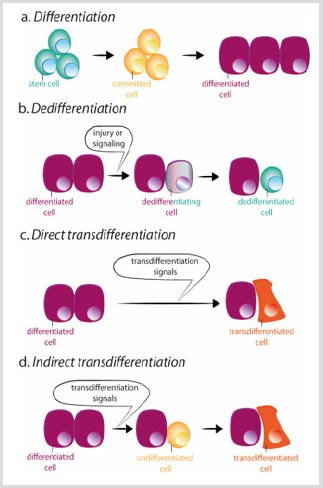
However, loss of cell identity can occur. Indeed, cells from Drosophila imaginal disc are able to transdetermine and acquire a new adult fate following transplantation [8]. In this situation, extracellular cues seem to reprogram some precursor or differentiated cells to acquire characteristics of either a more stem state or a new differentiated state. There are two mechanisms by which a cell can change its identity: dedifferentiation, and transdifferentiation. Dedifferentiation refers to the process by which a differentiated or committed cell acquires characteristics of a less mature cell [9] Figure 3b. The most dramatic example of dedifferentiation is the in vitro conversion of terminally differentiated cells into pluripotent cells (induced pluripotent stem cells, iPSCs), by the overexpression of a limited number of transcription factors [10]. Transdifferentiation, by contrast, occurs when a differentiated cell changes it transcriptional program and converts into another differentiated cell type. The process can occur through an intermediated step of dedifferentiation towards a less mature stage before the conversion into the new differentiated cell, or directly, without the intermediated stage [9] Figure 3c-d. The direct conversion of fibroblasts into myoblasts by the ectopic expression of MyoD is an example of the second process [11].
figure 3: Schemes of differentiation, dedifferentiation and trans differentiation.
a) During normal differentiation, stem cells give rise to committed cells which in turn differentiate in different cell types.
b) Dedifferentiation consists of the acquisition of stem cell properties by a differentiated cell. Trans differentiation can occur in a direct or indirect way.
c) During direct trans differentiation, a differentiated cell acquires the transcriptional program of another cell type, usually closely related, as for example exocrine to endocrine pancreatic cells, becoming a different differentiated cell.
d) During indirect trans differentiation, a differentiated cell dedifferentiates before adopting the new transcriptional program of the other cell type.
Dedifferentiation and transdifferentiation also occur in a natural way in response to an injury, or tissue loss [12]. Dedifferentiation, for example, occurs naturally during limb regeneration in the urodele amphibians. After limb amputation, cells adjacent to the wound dedifferentiate, forming a blastema that consists of undifferentiated cells that proliferate and eventually, redifferentiate into the same cell type to create all the components of the lost limb[12]. Natural transdifferentiation occurs indirectly: first, the cell dedifferentiates; and then the natural developmental program is activated, allowing the cell to differentiate into the new lineage[12]. Tsonis and collaborators described a natural mechanism of transdifferentiation in a newt. They found that when lenses are removed, pigmented epithelial cells from the dorsal iris transdifferentiate, and regenerate the missing tissue. To achieve this, pigmented epithelia cells must first dedifferentiate and proliferate to create new lens cells, and then differentiate into the mature cells of the lens [13]. In both situations – dedifferentiation and redifferentiation into the same cell type or transdifferentiate to a new cell type – a complex network of signaling pathways may control the transcriptional program acquired by each cell in the perfect time-point.
hus, the spatiotemporal control of gene expression is continuously required during animal homeostasis, and during a regenerative process. However, during regeneration, cells must re-adjust to the new situation, which requires making more profound decisions at a cellular level, often including processes of dedifferentiation and transdifferentiation that during homeostasis are scarce.
References
- Clevers H, Loh KM, Nusse R (2014) Stem cell signaling. An integral program for tissue renewal and regeneration Wnt signaling and stem cell control. Science 346(6205): 1-9.
- Leblond C P, Walker B(1956) Renewal of cell population 36(2): 255-276.
- Roostaee A, Benoit YD, Boudjadi S, Beaulieu JF (2016) Epigenetics in Intestinal Epithelial Cell Renewal. J Cell Physiol 231(11): 2361-2367.
- Ito K, Ito K (2016) Metabolism and the Control of Cell Fate Decisions and Stem Cell Renewal. Annu Rev Cell Dev Biol 32: 399-409.
- Simons BD, Clevers H (2011) Strategies for homeostatic stem cell selfrenewal in adult tissues. Cell 145(6): 851-862.
- Yang Y, Akinci E, Dutton JR, Banga A, Slack JM W, et al. (2013) Stage specific reprogramming of mouse embryo liver cells to a beta cell-like phenotype. Mech Dev 130(11-12): 602-612.
- Merrell AJ, Stanger BZ (2016) Adult cell plasticity in vivo: dedifferentiation and transdifferentiation are back in style. Nat Rev Mol Cell Biol 17(7): 413-425.
- Worley MI, Setiawan L, Hariharan IK (2012) Regeneration and transdetermination in Drosophila imaginal discs. Annu Rev Genet 46: 289-310.
- Raff M (2003) Adult Stem Cell Plasticity: Fact or Artifact? Annu Rev Cell Dev Biol 19: 1-22.
- Takahashi K, Yamanaka S (2006) Induction of Pluripotent Stem Cells from Mouse Embryonic and Adult Fibroblast Cultures by Defined Factors. Cell 126(4): 663-676.
- Tapscotr SJ, Davis RL, Thayer MJ, Cheng PF, Weintraub H, et al. (1988) MyoDi: A Nuclear Phosphoprotein Reqiring a Myc Homology Region to Convert Fibroblasts to Myoblasts. Science 242(4877): 405-411.
- Jopling C, Boue S, Izpisua Belmonte JC (2011) Dedifferentiation, transdifferentiation and reprogramming: three routes to regeneration. Nat Rev Mol Cell Biol 12(2): 79-89.
- Tsonis PA, Madhavan M, Tancous EE, Del Rio-Tsonis K (2004) Del Rio- Tsonis, K. A newt s eye view of lens regeneration. Int J Dev Biol 48 (8-9): 975-980.

 Mini Review
Mini Review