Abstract
Introduction: The management of infections of the vulva and vagina depend on the selection and administration of effective specific therapies that allow a fast treatment, enhancing the compliance of the patients.
Objective: The efficacy and safety of a new medicinal product containing 300mg tinidazole/200mg tioconazole/100mg lidocaine for the treatment against vaginal candida albicans, bacterial vaginosis and combined infections was evaluated upon 69 patients. Clinical controls, pH, 10% KOH probe, microscopical and microbiological analysis were performed at day 1, day 4, day 10 and at day 30 of the study. Success was defined as follows: 0 points = no symptoms, 1 point = mild symptoms, 2 points = moderate symptoms, 3 points = severe symptoms. Results: A complete clinical recovery at day 10 was observed in 54 (80.6%) patients. A clinical improvement in 12 (17.9%) and a failure in 1 patient (1,5 %). At day 30 a clinical complete recovery was observed in 58 (86.6%) patients, a clinical improvement in 7 (10.4%) and a failure in 2 patients (3 %).
Conclusion: This formulation (GynomaxXL), a vaginal suppository given at 3 consecutive days, is a highly effective and safe combination in the therapy of vulvovaginal candidiasis and bacterial vaginosis. Clinical trial registration number: GМXL-03-01.
Keywords:Candidiasis; Bacterial Vaginosis; Mixed Vaginal Infections; Tinidazole, Tioconazol
Introduction
Infection of the vulva and vagina are among the most common medical problems seen in general practice. There are three common types of vaginal infections: vulvovaginal candidiasis, bacterial vaginosis and trichomoniasis [1,2]. Vaginal infection due to simultaneous infection with at least two microbial organisms (mixed infection, e.g. bacterial vaginosis in a patient with vulvovaginal candidiasis) is also highly prevalent and make up approximately 30% of all cases [3-6]. Effective management of vaginal infections depends on accurate diagnosis, selection and administration of effective specific therapy and good compliance of the patient. Since classic signs and symptoms of common types of vaginal infections are often equivocal, the diagnosis cannot always be established based on clinical manifestations alone [2,6]. Laboratory support is necessary for a differential diagnosis or to confirm the clinical diagnosis. However, accurate identification of the causative microorganism is technically difficult, often affected by a wide variety of factors and therefore, clinical diagnosis of the cause of vaginosis is often incorrect [7].
Metronidazole 500mg and miconazole nitrate 100mg (Neo-Penotran→, Exeltis Turkey), was indicated for the treatment of vulvovaginal candidiasis, bacterial vaginosis and trichomonas vaginalis [8]. The recommended dosage schedule was one pessary at night and one pessary in the morning for seven days or one pessary at night for 14 days. Clinical studies carried out [8-12] have shown that with both dosage regimens, once daily for 14 days or twice daily for seven days, high clinical and microbiological cure rates were achieved with an excellent safety profile [8-11]. One other study [12], was also able to show a high efficacy for the immediate treatment of single and/or mixed vaginal infection. In addition, there appeared to be no significant difference regarding efficacy and safety between the 7-day and 14-day dosage regimens [12]. The next development was a combination of metronidazole (750mg) and miconazole nitrate (200mg). A tolerance, acceptability and absorption study [13] of this combination with and without lidocaine in healthy volunteers has shown that the pessaries are well tolerated. Ten subjects received metronidazole 750mg + miconazole nitrate 200mg + lidocaine 100mg for the first three days, and the same combination without lidocaine for the subsequent 4 days of pessary administrations. Systemic absorption of metronidazole revealed steady state plasma levels in a range approximately comparable with the standard 200mg oral dose. Lidocaine was minimally absorbed, and miconazole was not absorbed vaginally. Clinical results were very positive [14]. Moving forward a formulation containing the drugs tinidazole and tioconazole was developed. Duarte el al. was one of first in describing the efficacy of a combination of both drugs in the management of bacterial vaginosis and candida albicans [15].
Tinidazole
Tinidazole is effective against protozoa and anaerobic bacteria. Its antiprotozoal activity includes Trichomonas vaginalis, Entamoeba histolytica and Giardia lamblia. Tinidazole is effective against Gardnerella vaginalis and to most anaerobic bacteria (Bacteroides fragilis, Bacteroides melaninogenicus, Bacteroides spp., Clostridium spp., Eubacterium spp., Peptostreptococcus spp. and Veillonella spp.). The complete mechanism of action of tinidazole has not been fully clarified. The reduction of the nitro-group is mediated by a ferredoxin system and a low oxidation reduction potential which is only generated by anaerobic bacteria. This may be the reason for anaerobes having a higher tinidazole uptake than aerobes, although tinidazole can penetrate cell membranes of both types of microorganisms. The reduction process creates reactive intermediates and a diffusion gradient, which enhances tinidazole uptake. Although cross-resistance is common for 5-nitroimidazoles, 65% to 70% of Trichomonas vaginalis strains highly resistant to metronidazole have shown increased susceptibility to tinidazole [16].
Tinidazole is a nitroimidazolic compound chemically related to metronidazole. Anti-microbiotic effect of tinidazole emerges as a result of penetration to microorganism, replication leading to cellular mortality and formation of the complexes inactivating DNA by preventing proteic synthesis. It has a destructive activity against protozoa and anaerobics including trichomonas vaginalis, garnerella vaginalis, bacteroides melaninogenicos and other bacteria. Tinidazole is exhibited to be 16 times more potent than metronidazole in in vitro inhibition of trichomonas vaginalis and the adverse effects arising from the utilization of topical tinidazole are low [15]. In a comparative study between metronidazole and tinidazole done by Mattila et al. it could be demonstrated that at vaginal dosages of 500mg vaginal supossitiries with tioconazole a very low systemic amount of the substance was reached.
The Cmax was 1.0 +/- 0.2μg/ml and the AUC was 22,6 +/- 3.3μg x h/ml. these data were similar to those of 500mg metronidazole also as an vaginal ovulum [17]. Other assays showed that after oral administration, tinidazole is rapidly and completely absorbed. A bioavailability study of a tioconazole tablets was conducted in adult healthy volunteers. All subjects received a single oral dose of 2g (four 500mg tablets) of Tindamax→ following an overnight fast. Oral administration of four 500 mg tablets of tioconazole under fasted conditions produced a mean peak plasma concentration (Cmax) of 47.7 (±7.5)μg/ml with a mean time to peak concentration (tmax) of 1.6 (±0.7) hours, and a mean area under the plasma concentration-time curve (AUC, 0-°) of 901.6 (± 126.5)μg.hr/ml at 72 hours. The elimination half-life (t½) was 13.2 (±1.4) hours. Mean plasma levels decreased to 14.3 μg/ml at 24 hours, 3.8μg/ml at 48 hours and 0.8 μg/ml at 72 hours following administration [17,18 19]. Vaginal bioavailability of tinidazole is approximately 10% [16]. The Cmax was 1.0 (±0.2)μg/ml and tmax was 8.7 (±1.2) hours after application of intravaginal ovule containing 500 mg tinidazole on 6 healthy volunteers [17].
Distribution: Tinidazole is almost distributed into all tissues and body fluids and crosses the blood-brain barrier. The apparent volume of distribution is about 50 liters. Plasma protein binding of tinidazole is 12%. Tinidazole crosses the placenta and is secreted in breast milk [16].
Metabolism: Tinidazole is significantly metabolized in humans prior to excretion. Tinidazole is partly metabolized by oxidation, hydroxylation, and conjugation. Tinidazole is the major drugrelated constituent in plasma after human treatment, along with a small amount of the 2-hydroxymethyl metabolite [19].
Elimination: The plasma half-life of tinidazole is approximately 12-14 hours. Tinidazole is excreted by the liver and the kidneys. Tinidazole is excreted in the urine mainly as unchanged drug (approximately 20-25% of the administered dose). Approximately 12% of the drug is excreted in the feces [17,18,19]. Individually applied tioconazole is absorbed in low levels from the vaginal mucous.
Tioconazole
In healthy women who received a single 300 mg intravaginal dose of tioconazole as the commercially available 6.5% vaginal ointment, mean vaginal fluid concentrations of the drug 24, 48 and 72 hours after the dose averaged 703, 220 and 91μg/mL, respectively [15]. In another study in women with vulvovaginal candidiasis who received a single 300mg dose of tioconazole as a 6% vaginal ointment, mean concentrations of the drug in vaginal fluid were 104, 27, and 15μg/ml at 24, 48, and 72 hours, respectively [15]. Mean peak plasma concentration following the administration of 300mg single dose tioconazole ointment to women with candida vaginalis was found as 18 ng/mL and Tmax was calculated as 2-24 hours [15]. After vaginal insertion of a 300- mg ovule of tioconazole in 10 patients with vaginal candidiasis the mean of the concentration of tioconazole in vaginal fluid was 21.4mg/liter (range, 2.4 to 50mg/liter). The concentration appears to vary greatly from patient to patient, though eight of nine patients retained a level of >10mg/l of vaginal secretion at 24h [18].
Metabolism: Primary metabolite of tioconazole is glucuronide conjugate. Tioconazole does not appear to be metabolized in vaginal fluid, but a portion of the drug that is absorbed systemically flowing intravaginal application is metabolized [18]. One reported metabolite is formed from N-glucuronidation of a nitrogen on the imidazole ring, another is formed by O-dethienylation of a chlorothienyl group, hydration to an alcohol, and glucuronidation [19]. Tioconazole is an imidazole derivate having fungicide activity mainly acting by changing the normal membrane structure [18]. It presents a wide spectrum of activity as in vitro against dermatofits and candida albicans as well as against trichomonas vaginalis and gram-positive bacteria. Tioconazole is absorbed vaginally in minimum quantities, present a direct local effect on fungi and yeasts. Adverse effects of tioconazole are generally of mild to moderate intensity and burning, itching and allergic reactions are the most common described [18]. Jewons could demonstrate that the antifungal activity of tioconazole was higher than miconazole.
Tioconazole was superior to miconazole in reducing the amount of different yeast forms in vitro and in vivo showing almost no side effects. [18] Houang et al. could show that the systemic absorption of tioconazole after the use of a single vaginal ovulum containing 300 mg was really very low showing whether a local safety problem nor a systemic possible danger. After 8 hours only, a concentration of 21,2ng/ml was observed. This is far under the necessary MIC of 1,6 to 12,5mg/liter for candida albicans. After 48 hours some concentration of tinidazole could be measured in the blood, but at a very low level [20]. Therefore, the objective of this study was to evaluate the efficacy and tolerability of a new formulation with tinidazole and tioconazole ovules, administered once daily for three days for the treatment of bacterial vaginosis, candida albicans or mixed infections having in mind an improvement in patient compliance with a low side effect profile of the new drug formulation.
Material and Methods
Study Design
This was a multi-center, open-label study to evaluate the efficacy and safety of a medicinal product for intravaginal administration in the treatment of vulvovaginal candidiasis, bacterial vaginosis, trichomoniasis or mixed vaginal infections. The study was conducted in six primary-care centers located in Russia between 2017 and 2018. The trial was in accordance with the clinical study protocol (version 1.0 of 07/07/2016), the Principles of the Declaration of Helsinki of the World Medical Association (Fortaleza, Brazil, 2013) and the tripartite agreement on Good Clinical Practice (ICH E6). It was, authorized by the Ministry of Health of the Russian Federation (No. 790 of 10/11/2016) and approved by the Ethics Council No. 132 of 06/09/2016, and local ethical committees of the investigational sites. Study participants were informed about the study and provided written informed consent before enrolment. Clinical trial registration number: GМXL-03-01.
Introduction
Study Participants
Seventy women were recruited for the study. Women who participated in the study had to meet the following main inclusion criteria:
a) Age from 18 years to 50 inclusive.
b) To have at least one clinical sign and symptom of vulvovaginal candidiasis, bacterial vaginosis, trichomoniasis or mixed vaginal infection (candidiasis, trichomoniasis, bacterial vaginosis).
c) Positive result of one laboratory test (microscopy of Gram-stained smears, pH measurement, amine test, PCR-diagnosis) confirming the presence of pathogen of candidiasis, bacterial vaginosis, trichomoniasis, or mixed vaginal infection. Further inclusion criteria were the uncomplicated course of the disease that does not require systemic therapy, women had to have a preserved reproductive function, to be in the first phase of menstrual cycle after the end of menstrual bleeding, and to have negative urine pregnancy test as well as to agree to refrain from sexual relationships for the duration of the study. Main exclusion criteria were defined as the following: age < 18 years and > 50 years, known hypersensitivity or suspected hypersensitivity to one or more components of the medicinal product used in the clinical study, previous or present organic diseases of the nervous system, leukaemia, hemopoiesis disorders or blood dyscrasia, history of severe disorders of the liver and kidneys, history of epilepsy, current pregnancy or lactation period or virginity.
Furthermore, patients were not allowed to participate in case of recurrent course of vulvovaginal candidiasis (more than 4 times a year), the presence of a concomitant sexually transmitted infection (STI) in the diagnosis, a need for systemic therapy of the disease or in case the received systemic antibacterial medicinal products 4 weeks before being enrolled in the study. Women diagnosed with HIV infection, syphilis as well as hepatitis B and C virus were excluded as well.
Study Treatment
Patients diagnosed with a vaginal infection and enrolled into the study were treated once daily for three consecutive days with the vaginal ovulum GynomaxXL→ (manufactured by Exeltis Turkey). Gynomax XL→ is a vaginal suppository containing 300mg tinidazole, 200mg tioconazole, and 100mg lidocaine. Women inserted the ovulum each evening in the vagina. The treatment phase was followed by a follow-up phase of 27 days.
Study Endpoints
The primary efficacy endpoint was defined as frequency of cases of clinical recovery on Visit 3 (Day 10) when evaluating therapeutic results. Secondary efficacy endpoints were defined as
a) Frequency of cases of clinical recovery on Visit 4 (Day 30) when evaluating therapeutic results,
b) Frequency of cases of abatement/disappearance of clinical signs and symptoms of the disease on Visit 2 (Day 3), Visit 3 (Day 10) and Visit 4 (Day 30) in comparison with the baseline data of Visit 1 (Day 0-1).
c) Frequency of cases of elimination of the pathogen of infection with treatment, revealed by microbiological analysis, compared to baseline data of Visit 1.
Study Visits and Examinations
Each patient had 4 study visits: patient screening (Day 0-1), end of therapy (visit 1 (Day 4)), and two follow-up visits (Day 10 and Day 30). At each visit, gynecologic examination was performed including standard procedures of examination of external genitalia (skin and mucous membrane), speculum examination (mucous membrane of the vagina, cervix, pharynx, presence of discharge), bimanual examination (cervix, uterus, right and left appendages, septum recto vaginale).
Assessment of Clinical Signs and Symptoms of Vaginal Infection
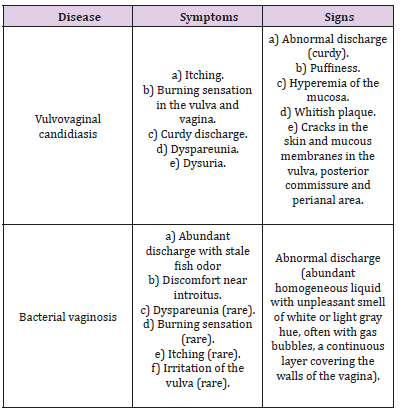
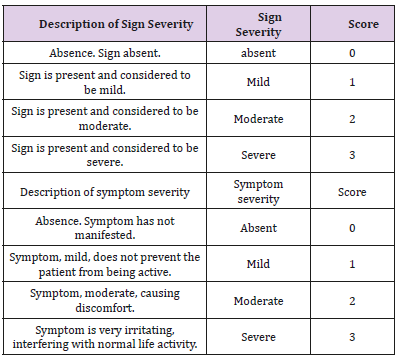
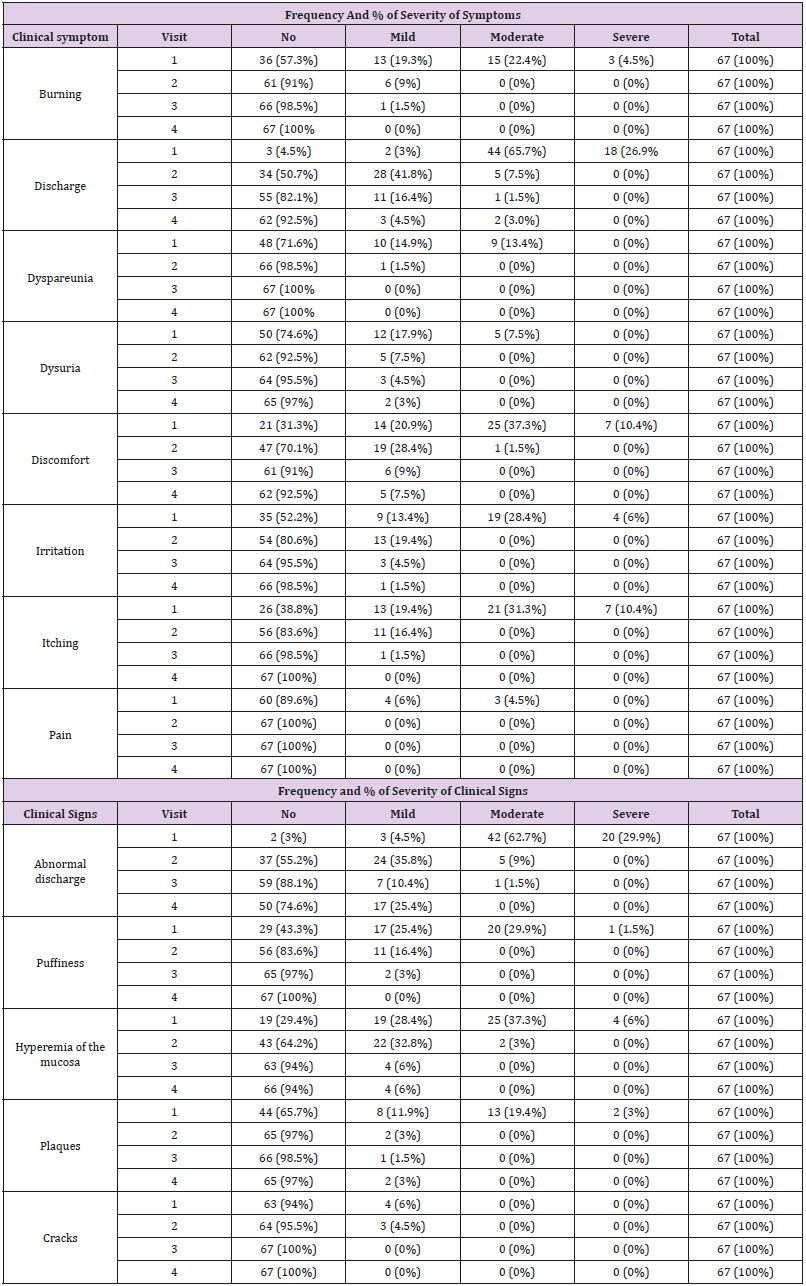
At all study visits clinical signs and symptoms of vaginal infections were assessed during gynecological examination and based on patient’s complaints. Table 1 lists common signs and symptoms that can be observed with vulvovaginal candidiasis and bacterial vaginosis and which have been included in the evaluation of the efficacy of therapy in accordance with their changes compared to the baseline. In the case of mixed vulvovaginal infections, these signs may overlap. The severity of clinical signs and symptoms and their dynamics was evaluated at each visit according to a scale given in Table 2. The results at each visit were compared and evaluated as abatement of a clinical sign or symptom if the severity of the symptom/sign has decreased by 1 point, as disappearance if the sign has disappeared (0 points), increase if the severity of the sign/ symptom has increased by 1 point, as without changes if the score has not changed since the previous visit.
Diagnosis of Type of Vaginal Infection
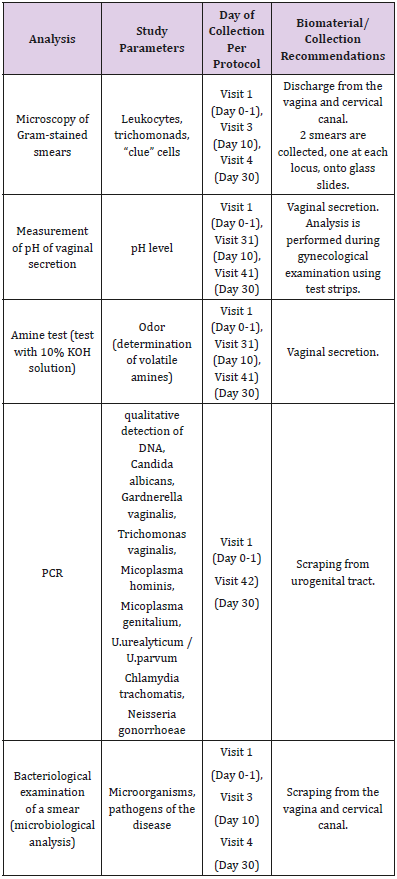
In order to confirm the diagnosis of vaginal infection and to identify the type of infection, the primary diagnosis was based on Gram microscopy, pH measurement, amine test, and PCRdiagnostics: An overview of all methods is provided in Table 3. Vulvovaginal candidiasis was diagnosed in case of detection of budding yeast cells and pseudmycelia using microscopy of Gramstained smears and when PCR was positive for DNA of Candida albicans. Bacterial vaginosis was diagnosed using the Amsel criteria including the detection of Gram-stained clue cells, a pH > 4.5, and a positive amine test – manifestation or exacerbation of an unpleasant odor of stale fish when adding 10% KOH solution with vaginal fluid. In addition, DNA of Gardnerella vaginalis was detected by PCR analysis. Trichomoniasis was diagnosed in case PCR analysis was positive for detection of Trichomonas vaginalis. If results of analyses (microbiological, PCR) revealed pathogens of other sexual transmitted infections (STIs), the patient was dropped out of the study and was not followed up. These tests were performed at all study visits. All necessary laboratory tests were performed in laboratories of investigational sites. For proper collection of biological samples, investigators used standard techniques for collecting biological samples of their respective investigational sites.
PCR Analysis
Results are presented as the frequency of cases (%) with or without growth of tested pathogen detected at baseline. In addition, the frequency of cases of elimination of the pathogen according to the conditional rate in the vaginal discharge of healthy women of reproductive age was investigated, since the detected microorganisms can be present in the results of a microbiological analysis in normal quantities in a healthy woman. As thresholds concentrations of 106cfu/mL gardnerella vaginals, 104cfu/mL Candida spp. and other anaerobic bacteria (from 103 to 105cfu/mL) were used.
Evaluation of Therapeutic Results
The dynamics of clinical signs and symptoms of the disease was recorded during the entire patient’s participation in the study at each visit, starting from the baseline data, obtained at the screening visit. Clinical signs and symptoms of the disease were evaluated at each visit according to a scale from 0 to 3 depending on the severity of the sign or symptom, in accordance with Table 2. Following scale was used: sign/symptom is absent – 0 points, mild – 1 point, moderate – 2 points, severe – 3 points. Therapeutic results on Visits 3 and 4 were evaluated based on results of clinical signs and symptoms in comparison with the baseline data of Visit 1.
Therapeutic results were categorized as follows:
a) Clinical recovery means a complete regression of clinical signs and symptoms of the disease,
b) Improvement means incomplete/partial regression of clinical signs and symptoms of the disease,
c) Absence of effect means absence of dynamics of clinical signs and symptoms of the disease or an increase in the intensity of one or more clinical signs and symptoms of the disease in comparison with previous visits.
Statistics
Statistical tests were chosen based on the type of data distribution and equality of variances. Analysis of normally distributed variables was done using parametric methods (ANOVA, t-Student test), otherwise nonparametric methods (Wilcoxon- Mann-Whitney test) were used. The same approaches were used with paired criteria when comparing the characteristics over time (paired Student’s t-test or paired Wilcoxon test). A comparative analysis of qualitative variables was done using the chi-square test and two-sided Fisher’s exact test. Results are presented in the form of a graph or table with comments (if necessary).95% confidence interval for proportions expressed by binary variables was calculated using Clopper-Pearson method.
Results
In total 70 women (mean age of 30,86 +/- 8,24 years, mean BMI of 22,44 +/- 2,29 kg/m²) were enrolled to the study. One woman dropped out from the study as she did not show up at Visit 2. As the patients took the medicinal product on their own it was unclear if this patient received any treatment, the women was excluded from the intention-to-treat (ITT) population. At Visit 2, two women were excluded from the study (refusal to participate in the study, exacerbation of a chronic disease). Hence, 67 women full-filled the study and were analyzed within the per-protocol population.
Diagnosis at Baseline
During the screening visit, the baseline diagnosis was obtained. 36 patients (52,2 %) were diagnosed with bacterial vaginosis, and 28 patients (40,6 %) with vulvovaginal candidiasis. In 5 patients (7,2 %) were enrolled with mixed infections. None of the patients was diagnosed with trichomoniasis.
Primary Endpoint of the Study
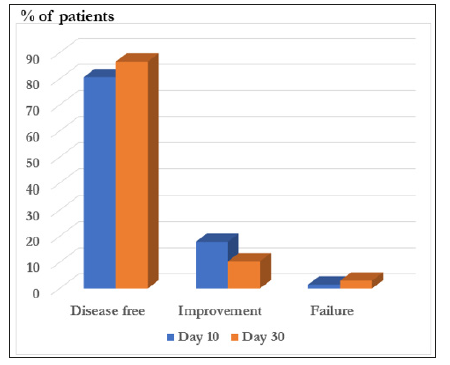
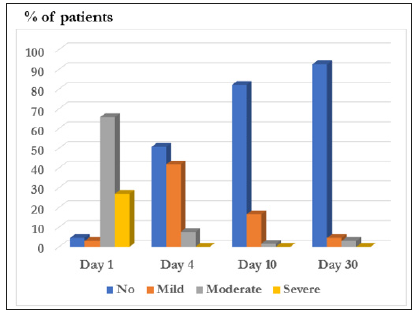
On day 10, in 54 (80.6%) patients a complete clinical recovery from the start of the therapy was observed. Improvement was achieved in 12 (17.9%) patients, while a failure of effect was recorded in 1 (1.5%) patient (Figure 1).
Secondary Efficacy Endpoints
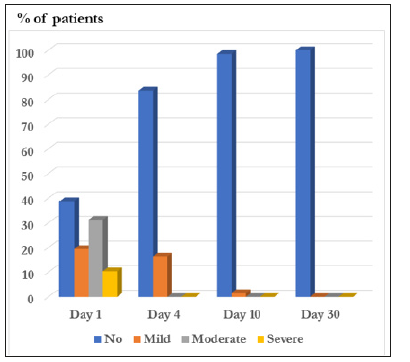
When evaluating the therapeutic results, the frequency of cases with a complete clinical recovery on day 30 from the start of the therapy was observed in 58 (86.6%) patients. Improvement was achieved in 7 (10.4%) patients. Absence of effect was recorded in 2 (3.0%) patients (Figure 1). The clinical symptoms (burning, discharge from the genital tract, dyspareunia, dysuria, discomfort in the introitus, irritation of the vulva irritation, pruritus, pain) as well as clinical signs (abnormal discharge, puffiness, hyperemia of the mucous membrane, plaques, cracks) of the disease were evaluated. The observed symptoms of the diseases diminished/disappeared during the investigation period, disappearing completely or almost completely at the end of the study (Visit 4). Similarly, the signs of the diseases clearly improved or disappeared completely or almost completely at the end of the study (Visit 4). The investigated signs were: burning, discharge, dyspareunia, dysuria, discomfort, irritation, itching, pain, abnormal discharge, puffiness, hyperemia of the vaginal epithelia, plaques and cracks (Figures 2 & 3) and (Table 4).
Microbiological Analyses
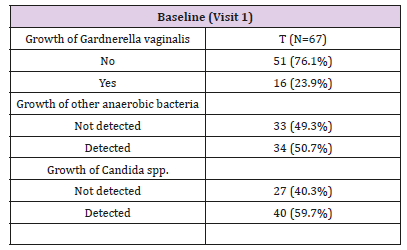
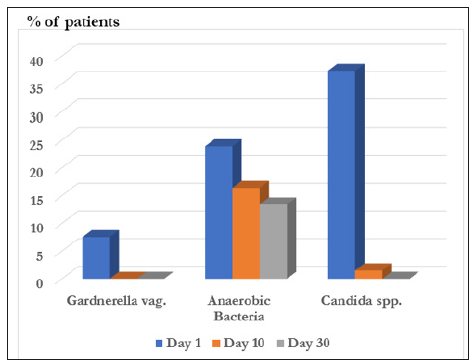
At baseline, Gardnerella vaginalis growth was initially detected in 16 (23.9%) patients, candida spp. growth, including candida albicans, – in 40 (59.7%) patients, growth of other anaerobic bacteria – in 34 (50.7%) patients (Table 5). The elimination analysis revealed the efficacy of the treatment. While before treatment, 7.5% of the patients had a growth of Gardnerella vaginalis > 106 after treatment there was no patient with such a Gardnerella vaginalis growth at day 10 and day 30. For candida albicans a growth above the level of > 104 was detected in 37,3 % of the patients before treatment and in 1,6 % of the patients after 10 days. At the last visit in none of the patient’s candida albicans growth was detected. For all the other anaerobic bacteria 23,9 % of the patients had values with more than 106 counts before treatment, while after 10 and 30 days 16,4 % and 13,4 % of the patients were above these levels (Figure 4).
Signs of Vaginal Candidiasis
In patients with vulvovaginal candidiasis results of Gramstained smears were analyzed. At baseline, white blood cells (leucocytes) were above the normal range in 20 (74.1%) patients. Ten (Visit 3) and 30 days (Visit 4) after treatment the number of patients with white blood cells above the normal levels decreased to 9 (33.3%) and 4 (14.8%) patients. At baseline, yeast cells and pseudomicelium were detected in 24 (88.9%) and 21 (77.8%) patients. These numbers also decreased over time (Yeast cells: Visit 3: 7 (25.9%) patients, Visit4: 1 (3.7%) patients; Pseudomicelium: Visit 3: 6 (22.2%), Visit 4: 1 (3.7%) patients.
Signs of Bacterial Vaginosis
The diagnosis of bacterial vaginitis was diagnosed by the investigation of the pH, Clue cells and an Amine test. pH values decreased during time from an initial mean value of 5.39 ± 0.59 to 4.28 ± 0.2 at Day 30. The Amine test was positive at baseline for all patients. After the treatment the test became negative at day 10 and day 30 for all patients. In 71.4% of the patients Clue cells were detected at baseline. The number of patients in which Clue cells could be detected decreased to 11.4% at day 10 and 5.7% at day 30.
Side Effects or Adverse Events
The safety assessment was analyzed based on the ITT population. There was no serious adverse event recorded during the study. Only 2 (2.9%) adverse events were recorded, which were not related to the investigational product. One patient was diagnosed with chronic cystitis and dropped out of the study. In one patient a relapse of bacterial vaginitis occurred.
Discussion
Vulvovaginal candidiasis and bacterial vaginosis are the most prevalent forms of vaginitis. Infection of the vagina with multiple pathogens is also very common; mixed forms of vaginitis, which complicate the diagnosis and treatment of vaginitis may be observed at a rate between 10 to 30% [3-6]. Bacterial vaginosis (BV) is a disease of the vagina caused by excessive growth of bacteria [21, 22]. Common symptoms include increased vaginal discharge that often smells like fish. The discharge is usually white or gray in color. Burning with urination may occur [23]. Itching is uncommon [21, 23]. Occasionally there may be no symptoms [24]. Having BV approximately doubles the risk of infection by a number of other sexually transmitted infections including HIV/AIDS [25,26]. It also increases the risk of early delivery among pregnant women [27,2 8]. BV is caused by an imbalance of the naturally occurring bacteria in the vagina [29, 30]. There is a change in the most common type of bacteria and a hundred to thousand-fold increase in total numbers of bacteria present [22]. Typically, bacteria other than Lactobacilli become more common [31]. Risk factors include douching, new or multiple sex partners, antibiotics, and using an intrauterine device among others [30].
However, it is not considered a sexually transmitted infection [32]. Diagnosis is suspected based on the symptoms and may be verified by testing the vaginal discharge and finding a higher than normal vaginal pH and large numbers of bacteria [22]. BV is often confused with a vaginal yeast infection or infection with Trichomonas [33]. BV is the most common vaginal infection in women of reproductive age [10]. The percentage of women affected at any given time varies between 5% and 70% [25]. BV is most common in parts of Africa and least common in Asia and Europe [25]. In the United States about 30% of women between the ages of 14 and 49 are affected [33]. Rates vary considerably between ethnic groups within a country [25]. While BV like symptoms have been described for much of recorded history, the first clearly documented case occurred in 1894 [21].Candida yeasts are generally present in healthy humans, frequently part of the human body’s normal oral and intestinal flora, and particularly on the skin; however, their growth is normally limited by the human immune system and by competition of other microorganisms, such as bacteria occupying the same locations in the human body [34]. Candida requires moisture for growth, notably on the skin [35]. In extreme cases, superficial infections of the skin or mucous membranes may enter the bloodstream and cause systemic Candida infections [35].
Factors that increase the risk of candidiasis include HIV/AIDS, mononucleosis, cancer treatments, steroids, stress, antibiotic usage, diabetes, and nutrient deficiency. Hormone replacement therapy and infertility treatments may also be predisposing factors [36]. Treatment with antibiotics can lead to eliminating the yeast’s natural competitors for resources in the oral and intestinal flora; thereby increasing the severity of the condition [37]. A weakened or undeveloped immune system or metabolic illnesses are significant predisposing factors of candidiasis [38]. Almost 15% of people with weakened immune systems develop a systemic illness caused by Candida species [39]. Diets high in simple carbohydrates have been found to affect rates of oral candidiasis [40]. Up to 75% of women reportedly have at least one episode of vulvovaginal candidiasis and 40-45% have 2 or more episodes during their lifetime but a small percentage 8up to 5% have recurrent vulvovaginal candidiasis [41]. results of several controlled clinical trials in non-pregnant women with uncomplicated vulvo vaginal candidiasis indicate that a single dose of tioconazole 6,5% vaginal cream results in a cure rate in 90- 96% and in mycological cure in 77-81 %of women at early follow up.
At late follow up the clinical and mycological cure rates were 71-90 and 54-77 % respectively. So, the efficacy even in a oneday treatment was proven long ago (421).Even if metronidazole remains the drug of choice for trichomoniasis and bacterial vaginosis the fact that treatment failure due to metronidazole resistant organisms is becoming more frequent has led to a change in the perception of the drugs of first choice (Dunne et al. 2003) [42]. Tinidazole has therefore not only been approved by the FDA in the management of this diseases but it appears to be better tolerated than metronidazole and has been therefore used successfully at higher doses in the management of this disease [43]. The combination of tinidazole and tioconazole represent an evolution in the management of these diseases. It was shown that with this combination, very high clinical and microbiological cure rates are achieved in patients with candida albicans and bacterial vaginosis and in mixed vaginal infections. As patient compliance is a major problem, and dosages like the oral established dosages should be used a combination of tinidazole (350mg) and tioconazole (100mg) was developed for once daily administration in the therapy of vaginitis.
This study, for the first time, could demonstrate that the new posology with the tioconazole and tinidazole pessaries, administered once daily for three days, provided effective and safe treatment in common types of vaginitis, as well as in mixed vaginal infections. The pessary was well tolerated and there was a drastic reduction in signs and symptoms of vaginal infections at the end of the three days’ treatment (visit 2) and follow up examination carried out at day 10 (visit 3) and day 30 (visit 4). Overall very high clinical and microbiological cure rates were observed.
Conflict of Interest
Pedro-Antonio Regidor and Manuela Sailer are employees of Exeltis Healthcare.
References
- Brocklehurst P, Mindel A (1991) Recurrent genital infections. Br J Sex Med 18: 54-57.
- Landers DV (1988) The treatment of vaginitis: Trichomonas, yeast, and bacterial vaginosis. Clin Obstet Gynecol 31(2): 473-479.
- Mardh PA, Tchoudomirova K, Elshibly S, Hellberg D (1998) Symptoms and sings in single and mixed genital infections. Int J Gynecol Obstet 63(2): 145-152.
- Govender L, Hoosen AA, Moodley J, Moodley P, Sturm AW (1996) Bacterial vaginosis and associated infections in pregnancy. Int J Gynecol Obstet 55(1): 23-28.
- Redondo Lopez V, Meriwether C, Schmitt C, Opitz M, Cook R, et al. (1990) Vulvovaginal candidiais complicating recurrent bacterial vaginosis. Sex Transm Dis 17(1): 51-53.
- Blackwell A (1987) Infectious causes of abnormal vaginal discharge - part two. Matern Child Health 12: 368-375.
- Ferris DG, Hendrich J, Payne PM, Getts A, Rassekh R, et al. (1995) Office laboratory diagnosis of vaginitis. Clinician-performed tests compared with a rapid nucleic acid hybridization test. J Fam Pract 41(6): 575-581.
- Morton O (1993) Neotran→-a new double -active pessary for the treatment of vaginitis. J Int Med Res 21: 36-46.
- Kükner S, Ergin T, Çiçek N, Uğur M, Yeşilyurt H, et al. (1996) Treatment of vaginitis. Int J Gynecol Obstet 52(1): 43-47.
- Altıntaş A, Embil K (1998) A new pessary for the treatment of vaginitis - clinical trial report (abstract 93.004). In: Altıntaş A, Embil K (Eds.) (8th Edn.) International Congress of Infectious Diseases, Boston, USA.
- Taner C, Yücel ES, İnce M, Kaçar A, Embil K (1998) Vaginitis and treatment (abstract 93.005). In Taner C, Yücel ES, İnce M, Kaçar A, Embil K (Eds.) (8th Edn.) International Congress of Infectious Diseases, Boston, USA.
- Özyurt E, Toykuliyeva MB, Danilyans IL, Morton O, Baktýr G (2001) Efficacy of 7-day treatment with metronidazole + miconazole (Neo- Penotran→)-a triple-active pessary for the treatment of single and mixed vaginal infections. Int J Gynecol Obstet 74(1): 35-43.
- Huseyinova L, Toykuliyeva MB, Özyurt E, Danilyans IL, Baktır G (2002) Tolerance, acceptability and absorption study with Neo-Penotran→ Forte-L and Neo-Penotran→ Forte in healthy volunteers (abstract 0158). In: Huseyinova L, Toykuliyeva MB, Özyurt E, Danilyans IL, Baktır G (Eds.) (10th Edn.) International Congress of Infectious Diseases, Singapore.
- Regidor PA, Ozyurt E, Toykuliyeva MB, Danilyans IL, Baktir G, et al. (2018) Treatment and prevention of trichomoniasis, bacterial vaginosis and candidiasis with a new 7-day regime containing metronidazole and meiconazole in a single vaginal pessary. IJMDAT 1(1): e118.
- Duarte G, Baracat EC, Wehba S (2016) Efficacy and tolerability of tioconazole/tinidazole combination in the treatment of vulovovaginitis. Femina 96(24): 895-904
- (2010) Tinidazole, Micromedex→ Healthcare Series [Internet database]. Greenwood Village, Colo: Thomson Reuters (Healthcare) Inc. Updated periodically.
- Mattila J, Männistö PT, Mäntylä R, Nykänen S, Lamminsivu U (1993) Comparative pharmacokinetics of metrinidazole and tinidazole as influenced by administration route. Antimicrobial agents and chemotherapy 23: 721-725.
- Jevons S, Gymer, GE, Brammer KW, Cox DA, Leeming MRG (1979) Antifungal activity of tioconazole (UK-20,349), a new imidazole derivate. Antimicrobial agents Chemotherapy 15(4): 597-602.
- Morton O (1993) Neotran→-a new double -active pessary for the treatment of vaginitis. J Int Med Res 21: 36-46
- Houang ET, Lawrence A (1995) systemic absorption and persistence of tioconazole in vaginal fluid after insertion of a single 300-mg tioconazole ovule. Antimicrobila Agents and Chemotherapy 27: 964-965.
- Borchardt, Kenneth A (1997) Sexually transmitted diseases: epidemiology, pathology, diagnosis, and treatment. CRC Press, Boca Raton, Florida: pp. 4.
- Donders GG, Zodzika J, Rezeberga D (2014) Treatment of bacterial vaginosis: what we have and what we miss. Expert opinion on pharmacotherapy 15(5): 645-657.
- Clark Natalie, Tal Reshef, Sharma Harsha, Segars James (2014) Microbiota and Pelvic Inflammatory Disease. Seminars in Reproductive Medicine 32 (01): 043-049.
- What are the symptoms of bacterial vaginosis?. 2013-05-21. Retrieved 3 March 2015.
- Kenyon C, Colebunders R, Crucitti, T (2013) The global epidemiology of bacterial vaginosis: a systematic review. American Journal of Obstetrics and Gynecology 209(6): 505-523.
- Bradshaw CS, Brotman RM (2015) Making inroads into improving treatment of bacterial vaginosis - striving for long-term cure. BMC Infectious Diseases 15: 292.
- Queena John T, Spong Catherine Y, Lockwood (2012) Queenan’s management of high-risk pregnancy: an evidence-based approach (6th ed.). Chichester, West Sussex: Wiley-Blackwell : pp. 262.
- (2015) What are the treatments for bacterial vaginosis (BV)?. National Institute of Child Health and Human Development.
- Bennett, John (2015) Mandell, Douglas, and Bennett’s principles and practice of infectious diseases. Philadelphia, PA: Elsevier/Saunders.
- (2015) Bacterial Vaginosis (BV): Condition Information. National Institute of Child Health and Human Development.
- Nardis C, Mastromarino P, Mosca L (2013) Vaginal microbiota and viral sexually transmitted diseases. Annali di Igiene 25 (5): 443-456.
- (2014) Bacterial Vaginosis - CDC Fact Sheet. Centers for Disease Control and Prevention.
- Mashburn J (2006) Etiology, diagnosis, and management of vaginitis. Journal of midwifery & women’s health 51(6): 423-430.
- Mulley AG, Goroll AH (2006) Primary Care Medicine: office evaluation and management of the adult patient. Philadelphia: Wolters Kluwer Health: pp. 802-803.
- Goehring, Richard V (2008) Mims’ medical microbiology. In Goehring, Richard V (Eds.) (4th Edn.). Mosby Elsevier, Philadelphia, USA: pp. 656.
- Nwokolo NC, Boag FC (2000) Chronic vaginal candidiasis. Management in the postmenopausal patient. Drugs Aging 16(5): 335-339.
- Bassetti M, Mikulska M, Viscoli C (2010) Bench-to-bedside review: therapeutic management of invasive candidiasis in the intensive care unit. Critical Care 14(6): 244.
- Odds FC (1987) Candida infections: an overview. Crit Rev Microbiol 15(1): 1-5.
- Choo ZW, Chakravarthi S, Wong SF, Nagaraja HS, Thanikachalam PM, et al. (2010) A comparative histopathological study of systemic candidiasis in association with experimentally induced breast cancer. Oncology Letters 1(1): 215-222.
- Akpan A, Morgan R (2002) Oral candidiasis. Postgraduate Medical Journal 78(922): 455-459.
- (2010) AHFS Drug Information. Published by Authority of the Board of the American Society of health System Pharmacists: pp. 3522.
- Dunne RL, Dunn LA, Upcroft P, O’Donoghue PJ, Upcroft JA (2003) Drug resistence in the sexually transmitted protozoan Trichonomas vaginalis. Cell res 13(4): 239-249.
- Sobel JD, Nyirjesy P, Brown W (2001) Tinidazole therapy for metronidazole-resistant vaginal trichomoniasis. Clin Infect Dis 33(8): 1341-1346.

 Research Article
Research Article