Abstract
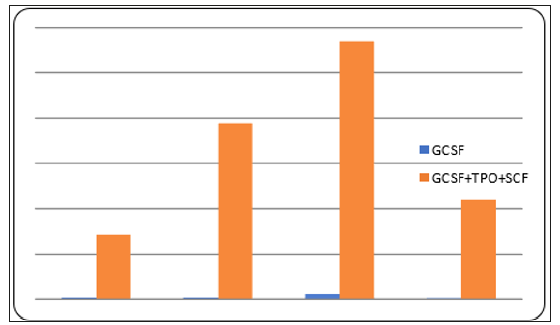
Stem cells are alternatives therapeutic treatment, mainly for diseases that cannot be treated by medical or surgical treatment. CD34+ hematopoietic stem cells which is isolated from autologous peripheral blood cell is a good source because it’s lowering immune rejection However, the limit volume of peripheral blood can be taken from a patient, limit the total number of CD34+ cell and therefore will be inadequate for therapeutic efficacy. In the previous addition of GCSF, TPO and SCF to CD34+ culture increased the number of CD34+ cell. But the addition of several cytokines will increase the cost of culture become a burden for patients who are seeking for stem cell therapy. In this study we examined the effect of GCSF on the expansion of CD34+ cell. For that purpose, we isolated and expanded CD34+ cell from peripheral blood using GCSF. As a control cultures, we used GCSF, TPO and SCF addition. Both culture was incubated for 10 days, and then counted the number of CD34+ cells. The results showed the amount of CD34+ cells can be increased only with GCSF. When compared to the control group, the number of CD34+ cells treated with GCSF were lower than which are an average 51,250 cells compare to 3,300,000 cells, respectively. There is a significant difference between the number of cd34 cells treated only with GCSF and the control group (p=0.04). Furthermore, the differentiation potential of CD34+ /GCSF expanded cells should be performed. In conclusion, GCSF alone has positive effect in increasing CD34+ cells number although the increase is lower compared to if all the treatment is combined.
Keywords:CD34+ cell; GCSF; Hematopoietic cell; Peripheral blood
Abbreviations:GCSF: Granulocyte Colony Stimulating Factors; CSF: Colony Stimulating Factors; HSC: Hematopoietic Stem Cells
Introduction
Human tissue has varying levels in regeneration capacity, even some of musculoskeletal tissues such as the cartilage and ligament have no ability to regenerate at all. In some condition when that tissues had trauma or diseased, they cannot regenerate. Until now, medical therapy has not been able to make growth or produce new tissue that tissue disorders are often end with surgical therapy such as replacement surgery. The solution is to make new tissue by regeneration therapy. Tissue engineering technique using stem cell could be the promising alternative therapy because of its ability to regenerate new tissue. Stem cells are cells that have ability to renew and multiply themselves and form cells and tissues structuring the organism body. Stem cells have some superior characteristics, namely plasticity produce regenerative tissue and prevent degeneration.
Stem cells can be categorized into embryonic stem cells and tissue stem cells. There are some limitations in using them such as ethical problem and teratoma risk. Tissues stem cells found in some tissues or organs. There are 2 types of tissue stem cells, mesenchymal stem cells and hematopoietic stem cells. Mesenchyme stem cells are potential sources of tissue engineering. However, the application of mesenchyme stem cells faces some limitations in sampling process and its nature. The sampling process causes pain and complication such as infection and sepsis. The other limitations are the characteristic of mesenchyme stem cells such as the number of cells, range of age, proliferation and its differentiation related to age of the donor. Hematopoietic stem cells are favorable because its characteristic, namely easy taking, low complication risk, and have differentiated potential without being affected by the age of the donor.
Hematopoietic stem cells are progenitor cells forming blood cells-lymphoid cells and myeloid cells. Cells, that come from bone marrow and blood respectively. Hematopoietic stem cells have pluripotent and plasticity so that they can form nonhematopoietic cells. The change of lineage is assumed to undergo some mechanism namely trans differentiation, dedifferentiation, and cell fusion. Hematopoietic stem cells can move toward the target tissues and form new cells and tissue. Major source of HSC is CD34+ cells, the other is CD133. Today, Hematopoietic Stem Cells (HSC) are clinically efficiently used for blood cancer therapy. More research indicates other uses of HSC for non-hematopoietic tissue such as for musculoskeletal tissue especially for ischemic chronic therapy in legs and osteogenesis imperfecta. Some researchers reported the use of hematopoietic stem cells for regeneration of non-hematopoietic tissue in experimental animals. Matsumoto reported the healing of rat’s femur bone fracture, through vasculogenic and osteogenic capabilities of CD34+ stem cells. Shi et al. reported the capability of CD133+ stem cells for the muscle tissue regeneration by healing of muscle injury in rats. Terayama reported the healing of osteonecrosis of rats’ hip joint. Basuki reported the healing of cartilage defect of the rat’s knee joint using CD34+ stem cell. It showed that CD34+ stem cell has capability of cartilage regeneration. These result indicate the differentiation potential that CD34+ cells have differentiation potential to become endothelial and osteoblasts cells.
CD34+ stem cell can be obtained by doing selection process of MNCs cells population. MNCs cells population taken by isolating the human body tissue such as solid or liquid. Skin and fat are examples from solid human body tissue whereas bone marrow, peripheral blood and cord blood are examples from liquid human body tissue. The process of isolating MNC cells from body liquids more preferable because of the easier process than isolating from solid human tissue. The collection process from bone marrow fluid and umbilical cord blood is inconveniences thus increased morbidity and easily contaminated. Therefore, collection process from peripheral blood human is safer and more comfortable for patients with some limitation [1]. The limitation of obtaining CD34+ cells from peripheral blood is the number of cells that can be used is not enough for therapeutically purpose [2-4].
The challenge is how to increase the number of CD34+ cells from peripheral blood selection for stem cell therapy. We need effective method to increase it. The previously methods are mobilization CD34+ from the bone marrow using cell growth factors namely GCSF (Granulocyte Colony Stimulating Factors) [5,6]. Colony Stimulating Factors (CSF), is hematopoietic growth factor, which plays a role in the regulation of bone marrow cell mobilization, proliferation and differentiation [7]. In fact, this method is done by give an injection to patient that feels uncomfortable to patient, another method is expansion CD34+ cells (In vitro) in a medium containing cell growth factors. The standard method of CD34+ cells expansion is cell expansion with multiple or combination of several cell growth factors, namely GCSF, SCF, TPO. GCSF has important role in CD34+ cells expansion [2,4,7,8]. CD34+ cells expansion with a mixture of several growth factors is less economical. the alternative is to use only one growth factor, namely GCSF. The purpose of the study was to determine the effectiveness of administration GCSF only compared with the administration of GCSF mixed with SCF and TPO.
Materials and Methods
Ethics Statement
Research was done at Stem Cell Research and Tissue Engineering Centre, Medical Faculty of University Pembangunan Nasional Veteran, Jakarta, Indonesia. All procedures were approved by Ethical Committee of University Pembangunan Nasional Veteran, Jakarta, Indonesia. Blood donor was volunteer who has been explained all of the procedures and use of their blood samples in this study and has knowingly signed the informed consent.
Blood Collection
Human peripheral blood was collected from 2 males, 30 years, healthy donors. Donors had no history of hepatitis, HIV, malignancy, or bone marrow disease and never had chemo or radiotherapy. Donors signed informed consent and undergone a preliminary blood examination to determine infection risk of Hepatitis and HIV, and both results were negative. All procedures in taking and examination of blood samples were conducted in Al Fauzan Hospital, Jakarta, Indonesia. About 100 ml peripheral blood was collected.
Peripheral Blood CD34+ Cells Selection
All collected blood were isolated with Ficoll gradient centrifugation and selected to CD34+ using Mini Macs column system. Blood specimens were diluted with PBS+ KCl solution, filtrated with Ficoll and centrifuged. Buffy coat layers were taken, washed and supernatant was removed until only MNC cells were available to be counted and viability checked. Afterward we used MACS isolation CD34+ kit (Militenyi) for CD34+ selection. MNC cells were washed and labeled by adding 300 μl buffer solution (auto MACS TM Rinsing Solution and MACS BSA Stock Solution, 20:1), FcR Blocking 100 μl, and CD34+ micro-beads 100μl, and the suspension was incubated for 30 minutes at 2-8°C. Subsequently, 10 mL buffer solution was added to cell suspensions and centrifuged. The supernatant was removed, and cells were re-suspended with 500 L buffer. Cells were separated with separator column. About 500 L of buffer was added into the column along with the cell suspension. The column was washed with 500 L buffer solution three times. CD34+ cells retained in the column were pushed with a syringe into a tube. Cell suspensions were centrifuged, the supernatant was removed, and cells were counted amount of cell and its viability.
CD34+ Cells Expansion
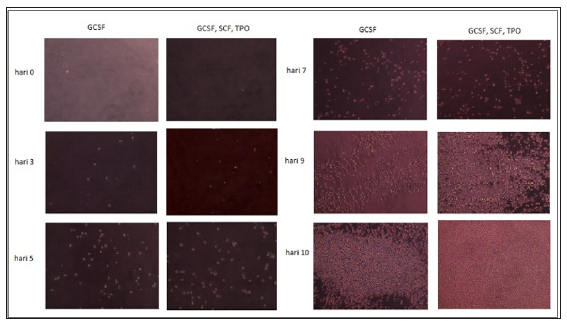
We prepared 24 well plates, 12 well plates for experimental group and 12 well plates for control group. We prepared two type of cell culture medium. Medium 1 : 10 4 CD34+ cells, GCSF (100 ng/ml, Miltenyi), Stem line II Hematopoietic Stem Cell Expansion medium (Sigma). Medium 2 : 10 4 CD34+ cells, in GCSF (100ng/ml, Miltenyi), TPO (100ng/ml, Miltenyi), SCF medium (100ng/ml, Miltenyi), Stem line II Hematopoietic Stem Cell Expansion medium (Sigma). We
put medium in well plate: medium 1 for experimental group and medium 2 for control group 1 (exin 12 well plate. We observed it for 10 days, and CD34+ cell was counted in day 10th.
 Research Article
Research Article