Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Mufeed Jalil Ewadh1* Ali Mohammed Jawad2 and Alaa Sadiq Al awad1
Received: September 24, 2017; Published: October 10, 2017
Corresponding author: Mufeed Jalil Ewadh, College of Medicine, University of Babylon, Hilla, IRAQ
DOI: 10.26717/BJSTR.2017.01.000421
Cancer is a group of diseases that cause cells in the body to change and grow out of control. Most types of cancer cells eventually form a lump or mass called a tumor, and are named after the part of the body where the tumor originates. Breast cancer begins in the breast tissue that is made up of glands for milk production, called lobules, and the ducts that connect the lobules to the nipple. The remainder of the breast is made up of fatty, connective, and lymphatic tissues [1]. Glutathione (GSH) is a tripeptide of glycine, cysteine and glutamic acid that is found in high concentration intracellularly, being the most abundant low molecular mass thiol [2]. Synthesis of GSH requires the consecutive action of two enzymes, first glutamate-cysteine ligase (GCLC) that conjugates glutamic acid and cysteine forming gamma-glutamyl cysteine.
This compound containing cysteine with a sulfhydryl group (SH) is responsible for the antioxidant activity of GSH. The second reaction is the binding of gamma glutamyl cysteine with glycine by the enzyme glutathione synthetase (GSS), giving rise to the tripeptide gamma glutamyl cysteine glycine-glutathione. When there is an excessive production of reactive oxidative species (ROS), the antioxidant defense system is triggered, which is of great importance in the physiopathology of diverse cancer types, including breast cancer [3,4]. The consequence of this process is the loss of cellular function and progression towards cell death. Moreover, oxidative stress leads to DNA damage and mutations in tumor suppressor genes, events that can be important in the initiation of carcinogenesis.
The experimental work was done in Department of Biochemistry, College of Medicine, and University of Babylon.
Patients: Patient groups consist of (80) samples from women with breast cancer, divided into two groups. First groups (A) consist of (n=40) sample women with breast cancer without chemotherapy while second groups (B) were (n=40) sample women with breast cancer after chemotherapy treatment. The age of women was ranged from (37 - 85) years. All samples were collected from Oncology Center of Marjan Teaching Hospital in Hilla City, Based on the history: patients with positive family history of breast carcinoma, smokers, received hormonal therapy, chronic disease (e.g. diabetes mellitus, liver dysfunction, rheumatoid arthritis) were excluded from the study. This described in (Table 1).
Controls :Control groups (C) consist of (n= 40) samples of healthy women. They were collected from medical staff who were free from signs and symptoms of breast cancer , age ranged from (35 - 85) years, all of them were non-smokers , free from DM,hypertension and no family history of breast cancer This described in (Table 1).
Samples Collection :Blood sample were obtained from subject group in fasting state .Blood put in gel tube and separated at 3000 xg for 10 min at 4ºC to obtain serum .Consequently , serum was divided into aliquots in eppendorf and stored at ( -20ºC) until time of analysis (Table 1).
Determination of Glutathione in Serum using Reversed- Phase High-Performance Liquid Chromatography: This method is used in particular for the detection of glutathione in the serum and also used for the detection of sulfur glutathione disulfide (GSSG) based on a derivatization glutathione with orthophthalaldehyde (OPA) at pH 12, This measurement is used High- Performance Liquid Chromatography Technique of Fluorescence Detector (HPLC-FLD), which is characterized by high sensitivity, high specificity , and high selectivity for many of the compounds and in particular the glutathione [5].
HPLC Method: Samples were analyzed by High Performance Liquid Chromatography (HPLC) system, model Shimadzu 10AVLC equipped with binary delivery pump model LC-10AV. The eluted peaks were monitored by RF-20A prominence fluorescence detector. The condition of separation was listed in (Table 1). Standards of suspected compound were run similarly for the identification, quantification, and concentration of each isolated compound [6,7] (Table 2).
Table 1 : Patients and Control groups.
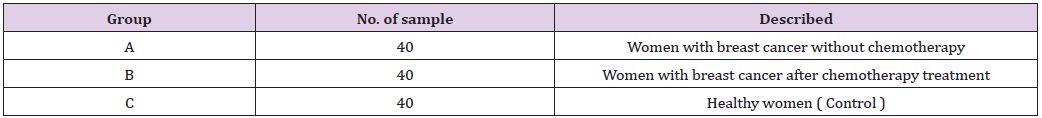
Table 2: Separation Conditions of High Performance Liquid Chromatographic.
Calculation : The area under the peak is used for calculating the concentration of a sample as the following formula:-Concentration of sample (μg/ml) = (the area of the sample / area of the standard) × Standard Conc. × Dilution factor
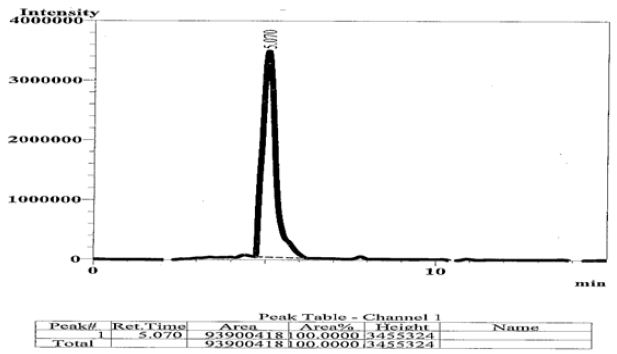
In this study, it was depended on the division of models, as described in (Table 1). It has been developed thorough examination to assess the GSH concentration in serum of breast cancer patient. Figure 1 shows that a complete baseline separation was obtained within glutathione by HPLC [8,9]. Cancer multifaceted complex disease affecting various different cancer patients in ways that demonstrate the imbalance in the control mechanisms that ensure the proper functioning of cells. An increase in the levels of free radicals may lead to a reduction in various cellular defense systems and the damage is irreversible, to cell death. Resulting in a lower concentration of antioxidants [10,11] .
Figure 1 : HPLC Chromatograph of Standard GSH , its Retention Time (RT= 5.07).
The (Mean ± SD) and of reduced glutathione in serum had decreased in the patient with breast cancer without chemotherapy in comparison to that of control group, while the (Mean ± SD) and of reduced glutathione in serum had increased in the patient with breast cancer after chemotherapy treatment in comparison to that of control group, (Table 3). According to P-value (p<0.05), there was a significance difference between the Mean of GSH in serum of patients compared to Mean of GSH in serum of controls (Figure 2).
Figure 2 : GSH Concentration in Serum of Breast Cancer Patients without and After Chemotherapy Compared to Healthy Women.
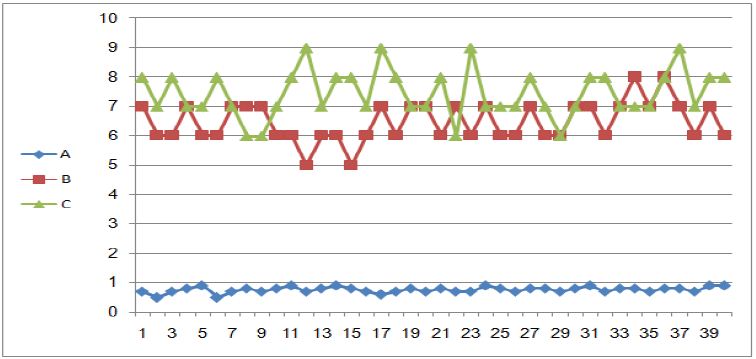
Table 3 : GSH of Breast Cancer with Healthy Control Group.

These finding may be long to that after chemotherapy which prevent DNA damage, thus preventing the proliferation of cancer cells from growth and thus return to normal cell, which reduces the presence of free radicals and increase the concentration of antioxidants, this is what was observed in the concentration of glutathione levels after taking chemotherapy [12]. Serum GSH and found to be significantly decrease in breast cancer. This was in agreement with other studies such as Prabhu [13], and Ewadh [14].
This study suggests the benefit of measuring serum GSH activity to assess BC. Simply measuring GSH activity along with the cost-effectiveness of giving additional feature to consider GSH as a sign of the tumor marker in the breast cancer. This study found that the chemotherapy prevents DNA damage in women with breast cancer, raise antioxidant levels mainly in glutathione levels after chemotherapy.


