Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Vongjen NP1*, Ombugadu A2, Adejoh VA2 and Njila HL3
Received: December 05, 2023; Published: December 18, 2023
*Corresponding author: Vongjen NP, Staff Officer Marine Police, 2nd Floor, Louis Edet House, Force Headquarters, Shehu Shagari Way, Area 11, Garki, Abuja, Nigeria. Email: daffason82@gmail.com, Tel: +2348060006725
DOI: 10.26717/BJSTR.2023.54.008515
Soil is one of the interfaces through which essential ecological processes take place. Therefore, this study was narrowed to determine the seasonal arthropod taxonomic composition in soils from some locations in Maiduguri, Borno State, Nigeria between January and December 2015. Samples were collected from four selected dumpsites in the following locations: Wulari, Abaganaram, Bulunmkutu and Bolori using a stratified random sampling design. Arthropods were extracted from the soil and identified using standard identification keys. A total of 748 arthropods were extracted and identified. The order Acarina were significantly (P = 0.02885) most abundant during the wet season (25.7%) and dry season (22.1%) respectively. There was no significant difference (P = 0.3899) in the abundance of arthropods along gradient during the dry season. In this study, there was no significant difference (Wet Season: P = 0.05458; Dry Season: P = 0.5351) in QBS index of arthropods in both wet and dry season respectively. The findings presented in this study are anticipated to aid in establishing foundational data on the arthropod fauna within the Maiduguri metropolis. Crucially, this data will serve as a valuable tool for monitoring potential shifts in both the abundance and diversity of soildwelling arthropods in Maiduguri metropolis.
Keywords: Arthropods; Composition; Soil; Taxonomy; Dumpsites; Gradients; Maiduguri
Abbreviation: QBS: Soil Biological Quality
Soil serves as a crucial interface facilitating essential ecological processes. It serves as the foundation for the root systems of terrestrial plants, providing them with vital nutrients [1]. Additionally, soil plays a pivotal role in various ecosystem functions, serving as an indispensable element in the biosphere. It regulates plant productivity, facilitates the degradation of organic matter, and contributes to nutrient cycles [2,3]. City and town soils offer a range of ecosystem services to urban dwellers. Nevertheless, urbanization impacts these soils and their ability to deliver ecosystem services directly due to human- induced disturbances [3,4]. Human activities such as industrial operations, construction, and various other practices are linked to diverse effects on the soil system. These effects encompass chemical pollution, such as contaminants like heavy metals, the transformation of native habitats into different land uses, and the fragmentation and loss of habitats [5]. An increasingly important field in applied entomology and ecology involves employing arthropods as indicators of environmental quality [6-9]. Arthropods, especially in the context of soil environmental quality, have shown promise as bioindicators, with research indicating their presence even in highly disturbed soils [10]. These creatures are highly suitable for investigating the effects of human activities on the environment, making them valuable candidates for such studies [4]. Soil in urban areas is highly susceptible to human-induced disruptions, particularly from chemical pollutants such as heavy metals [11].
These pollutants have the potential to harm the ecological systems that play a crucial role in providing essential services to cities, including air and water purification, as well as the preservation of biodiversity. Current conventional methods for environmental monitoring are not only expensive and time-consuming but also indirect in their approach. Utilizing arthropods as bioindicators offers a promising alternative for a quicker, more cost-effective, and more direct assessment [12]. With this objective, the focus of this study was narrowed to analyze the taxonomic composition of arthropods in soils at various locations in Maiduguri, Borno State, Nigeria.
Study Area
The study was conducted within the city of Maiduguri, Borno Sate, Nigeria.
Sample Collection: Samples were collected between January and December 2015. The collection of samples followed a stratified random sampling design at four dumpsites (Wulari, Abaganaram, Bulunmkutu, and Bolori), in accordance with the methodology outlined by [13,14]. From each dumpsite, four samples were obtained along an elevated gradient of pollution points, specifically at distances of 0 meters, 25 meters, 50 meters, and the final point (control site) positioned 100 meters away from each dumpsite. This resulted in a total of 16 samples per sampling season. Over the entire data collection period, a cumulative total of 32 samples were gathered. Sampling was stratified based on both season (dry and wet) and the distance from the pollution point source (waste dump) [14].
Extraction of Arthropods from the Soil: The arthropods were obtained from the soil samples by employing Tullgren’s funnel, a variant of the Berlese funnel extractor [15,16].
Identification of Extracted Specimens: Following the completion of all extractions, the preserved soil arthropods from each specimen bottle were sequentially emptied into a Petri dish, screened, and then identified using a high-resolution dissecting microscope. Utilizing entomological keys provided by Borror and Delong [17], Popov and Stojanova [18], and Kaur [19]. The identified arthropods were categorized into various Orders.
Data Analysis: Data was analyzed using R Console software version 3.2.2. One way analysis of variance (ANOVA) was used to compare the mean of relative morpho-types abundance of arthropods and soil biological quality (QBS) index in relation to gradients in dumpsite, seasons as well as across locations. The level of significance was set at P < 0.05.
Abundance of Soil Arthropod Groups at Dumpsite along Gradients
A sum of 748 arthropods were extracted and identified from the 32 samples collected at different gradients throughout the entire duration of this study. These include arthropods belonging to order acarina, aranae, chilopoda, coleoptera, collembolla, diplura, diptera, hymenoptera, lepidoptera and thysanoptera. Those arthropod group that could not be identified at the course of the study were termed to as ‘others’. A significant difference (F12 = 4.262, Adjusted R2 = 0.3948, P = 0.02885, Figure 1a) was observed in the abundance of arthropods along gradients during the wet season. The highest abundance was observed in the acarina order (25.7%), followed by the hymenoptera order (11.7%). In contrast, the diplurans (3.6%) were the least encountered during the wet season (Table 1). The unidentified arthropod group showed an abundance of 0.5%.
Table 1: Relative Abundance of Soil Arthropod Groups Occurring from an Elevated Gradient Distance in Dumpsite during Wet Season.

Table 2: Relative Abundance of Soil Arthropod Groups Occurring from an Elevated Gradient Distance in Dumpsite during Dry Season.
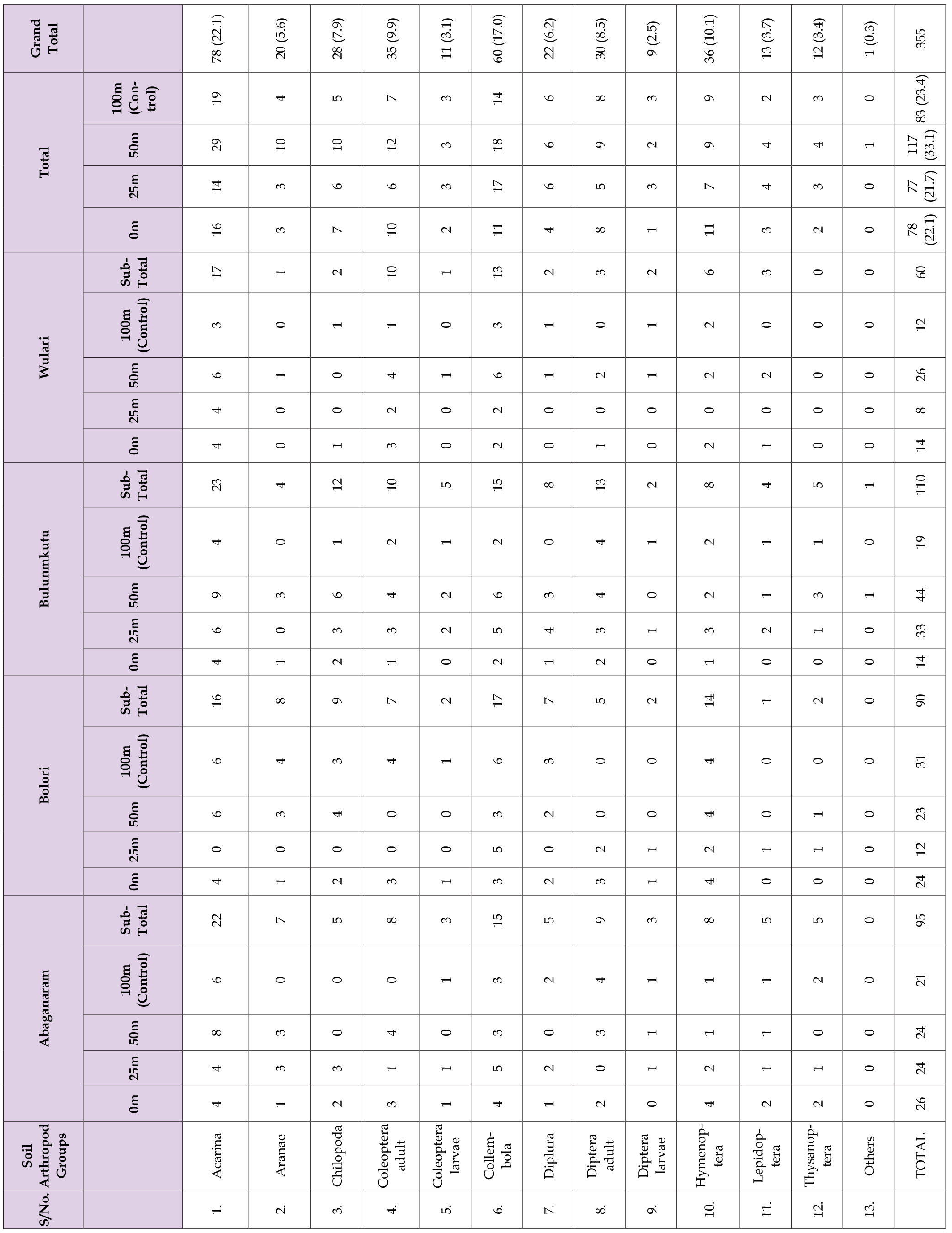
There was no significant difference (F12 = 1.092, Adjusted R2 = 0.01809, P = 0.3899, Figure 1b) in the abundance of arthropods along gradients during the dry season. The acarina order (22.1%) exhibited the highest arthropod abundance, followed by the collembola (17.0%), while the thysanoptera (3.4%) had the lowest abundance during the dry season (Table 2). The unidentified arthropod group displayed an abundance of 0.3%. Table 1 displays the proportional distribution of soil arthropods across an elevated gradient near dumpsites in the wet season. A total of 393 arthropods were gathered from the examined dumpsites in the selected locations.
There was no significant difference (Abaganaram: F = 0.216, df = 48, P = 0.885; Bolori: F = 0.989, df = 48, P = 0.406; Bulunmkutu: F = 0.558, df = 48, P = 0.645; Wulari: F = 1.114, df = 48, P = 0.353) in the abundance of soil arthropods observed at different gradients in dumpsites at the selected dumpsites during the wet season. Table 2 show the relative abundance of soil arthropod occurring from an elevated gradient from dumpsites during the dry season. A total of three hundred and fifty-five (355) arthropods were collected from the dumpsites examined in the selected locations. There was no significant difference (Abaganaram: F = 0.1000, df = 48, P = 0.959; Bolori: F = 1.372, df = 48, P = 0.263; Wulari: F = 2.072, df = 48, P = 0.116) in the abundance of soil arthropods observed at different gradients at the selected dumpsites during the dry season. However, there was a significant difference (F = 4.556, df = 48, P = 0.007) in the abundance of soil arthropods at varying gradients at Bulunmkutu dumpsite. Further tests (Post Hoc Test) shows that the mean abundance at 50m was significantly higher than that of 0m and 100m but was not significantly different from that of 25m.
Arthropods Soil Biological Quality (QBS) Index
In this study, there was no significant difference (Wet Season: F12 = 3.373, Adjusted R2 = -0.04928, P = 0.05458, Figure 2a; Dry Season: F12 = 0.7652, Adjusted R2 = 0.3219, P = 0.5351, Figure 2b) in QBS index of arthropods in both wet and dry season, respectively.
Among the 748 arthropods surveyed, identified, and categorized in this investigation, those belonging to the acarina order emerged as the most prevalent. The decomposition of organic matter is significantly enhanced by mites, millipedes, earthworms, and termites. Moreover, ants, termites, earthworms, and other soil macrofauna actively shape the soil environment by creating channels, pores, aggregates, and mounds, thereby influencing the transportation of gases and water in the soil and modifying microhabitats for fellow soil organisms [20,21]. Consequently, the considerable variability observed in the abundance of soil arthropods in this study implies that acarina order arthropods exhibit tolerance to a broad spectrum of soil properties, rendering them less indicative of disparities in soil conditions [22,23]. This holds true for collembolas, hymenopteras, and coleopteras, which were equally abundant across various levels of the examined gradients.
It is noteworthy that while arachnids (order Araneae) and centipedes (Chilopoda) displayed a low population count, beetles (Coleopterans) and flies (Dipterans) exhibited high numbers. This inverse correlation might be attributed to competition for food and interactions between these taxa, as certain dipterans and coleopterans are known to prey on arachnids and centipedes [24,25]. While the Abaganaram dumpsite exhibited the highest abundance of soil arthropod groups and QBS values at 100m, a notable similarity in the diversity of soil arthropods was observed across all locations during the rainy season in this study.
Therefore, the uniformity observed in the soil arthropod groups across dumpsites at varying distances from an elevated gradient in all study locations may be associated with consistent microclimatic conditions prevailing during the rainy season. As suggested by Schröter et al. [26], Hågvar and Klanderud [27], Kardol et al. [28], Lindroth [29] and Menta and Remelli [30], microclimatic factors significantly influence the abundance of soil arthropods. Another contributing factor could be the effluent activity along the elevated gradient, evenly distributing a substantial amount of pollution along the gradients. Furthermore, the heightened reproductive activity during the rainy season, leading to increased diversity in soil arthropods, might elucidate the lack of variation observed along the elevated gradient distance from the studied dumpsites [31]. Moreover, it is plausible that the soil arthropods encountered in this investigation have acclimated to the seasonal fluctuations in soil nutrient concentrations [32,33], heavy metal levels [34], and physicochemical parameters [35] observed across varying distances in dumpsites at the study locations. This finding is consistent with Kardol et al. [28] discovery of no discernible variation in the abundance of soil arthropods across their study sites. Nevertheless, it diverges from the observations of Zhang et al. [36], who noted a significant increase in the abundance of soil nematodes with greater distance in their study. In the dry season, the highest QBS was observed 50 meters away from the Bulunmkutu dump site. Notably, the Bulunmkutu dump site exhibited a significantly greater abundance of soil arthropods at an elevated gradient distance in comparison to all other locations studied.
This correlation could potentially be attributed to the favorable microclimatic conditions of the soil specifically at the 50-meter mark from the Bulunmkutu dump site. Furthermore, the absence of heavy metal detection at this 50-meter distance from the Bulunmkutu dump site may contribute to the markedly higher abundance of soil arthropods observed at that location. The presence of heavy metals, such as mercury, in soil can alter both the physical and biological characteristics of the soil, as indicated by Tchounwou et al. [37] and González et al. [38].. Mercury is a known active ingredient in synthetic pesticides, which are acknowledged for their harmful effects on arthropods. This, in turn, can directly impact the composition and abundance of soil arthropods, as highlighted by Odumo et al. [39] and Buch et al. [40]. The consistent lack of diversity in the abundance of soil arthropod groups observed across an elevated gradient in the remaining dumpsites during the dry season may be linked to a uniform distribution of nutrients along the gradients. During this study, nutrients were evenly dispersed from 0m to 100m throughout the dry season. Consequently, it is reasonable to infer that this uniform distribution plays a role in the abundance of soil arthropods at these study sites. Furthermore, the absence of variation during the dry season at most dumpsites, except at Bulunmkutu, might be attributed to a period of non-reproduction and low activity. This factor could result in a disparity leading to a distinction in morphotype composition among the recorded arthropod groups in this research. The lack of variation could also be ascribed to seasonal changes and the timing of sampling [41]. Additionally, it could be linked to similar occurrences of heavy metal concentrations resulting from effluent activities at the various dumpsites, likely established during the rainy season. These findings contradict the findings of Schröter et al. [26], Hågvar and Klanderud [27], Kardol et al. [28], Lindroth [29] and Menta and Remelli [30], who reported significant variations in QBS values across seasons.
Throughout both seasons, there was consistent soil arthropod group abundance, showing no significant variation between rainy and dry seasons. However, on average, arthropods were more abundant during the wet season compared to the dry season, except at Bulunmkutu dump sites where soil arthropods were more abundant in the dry season. The higher abundance of soil arthropods during the rainy season is likely associated with increased soil moisture resulting from rainfall. van Straalen [43] suggested that continued rain enhances soil moisture, promoting the proliferation of soil arthropods due to nutrient availability. Furthermore, the rainy season provides favorable conditions such as abundant high-quality food resources, optimal temperature, soil moisture, and radiation, all of which contribute to the thriving of soil arthropods [44,45]. This finding aligns with the results of Wolters [46], Pizl et al. [47], and Santorufo et al. [48], who also reported no significant differences in their respective studies.
This study revealed a relatively high population of soil arthropods (748) in dump sites and along its gradient away from point source. The order Acarina was the most abundant in both seasons all year round, wet season (25.7%) and dry season (22.1%). Arthropods abundance along gradient during the dry season lacked significant variation. Also, no difference was observed in QBS index of arthropods in wet season as well dry season. The comprehensive investigation of soil-dwelling arthropods in Maiduguri, Borno State, with regard to population abundance and diversity, is still pending to some extent. This study marked one of the initial endeavors to explore the variety and population of soil-dwelling arthropods in the Maiduguri metropolis. The data produced by this study would be valuable in establishing a preliminary baseline for soil-dwelling arthropod groups. For future research, it is crucial to perform year-round sampling and employ both morphological and molecular techniques to identify arthropods at the species level.
All the authors declared no conflict for the publication of this article.


