Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Jianwei Wu1, Jing Zhang1, Xiaomei Yi2,3 and Jianwei Wu1*
Received: August 24, 2023; Published: September 19, 2023
*Corresponding author: Jianwei Wu, Department of Medical Technology, Gannan Healthcare Vocational College, Ganzhou, China
DOI: 10.26717/BJSTR.2023.52.008313
Tumor progression is one of the major challenges in cancer treatment, and macrophages, as an important component of the immune system, have been extensively studied for their role in tumor immunomodulation. Exosomes are a group of small vesicles secreted by cells containing a variety of biologically active molecules, such as proteins, nucleic acids, and lipids. Recent studies have shown that exosomes serve as an important messenger for macrophages, which participate in the regulation of tumor growth, metastasis, immune escape and drug resistance by releasing exosomes. In addition, macrophage-derived exosomes play an influential role in tumor immunotherapy. They can transport immunomodulatory molecules, such as cytokines and antigens, which interact with tumor cells and other immune cells to facilitate immune recognition and killing of tumors. Macrophage-derived exosomes also modulate tumor-induced immunosuppression and enhance anti-tumor immune response. Consequently, the role of macrophage-derived exosomes in tumors may be dual, with both antitumor and tumorpromoting effects. A better understanding of macrophage-derived exosomes will provide new strategies to improve the efficacy of anticancer therapies. In this review, we focus on the biogenesis of macrophagederived exosomes and the mechanisms by which they mediate cancer progression, including on tumor cell proliferation, angiogenesis, invasion and metastasis, immune evasion and drug resistance. Finally, we discuss the potential clinical application of macrophage-derived exosomes as a promising strategy for tumor immunotherapy, hoping to provide new ideas and targets for further exploration of tumor pathogenesis and therapeutic approaches.
Keywords: Macrophage; Exosome; Tumor; Generation; Molecular mechanism; Therapy
Abbreviations: TME: Tumor Microenvironment; TAMs: Tumor-Associated Macrophages; MDE; Macrophage-Derived Exosomes; lncRNA: Identified Long Chain Non-Coding RNA; MVBs: Multivesicular Bodies; ILVs: Intraluminal Vesicles; ESCRT: Endosomal Sorting Complex Required for Translocation; VTA1: Vesicle Transport 1; ALIX: Apoptosis-Associated Gene 2-Interacting Protein X; VPS4: Vesicle Sorting Protein 4; LAMP1: Lysosome-Associated Membrane Protein 1; HEK293: Human Embryonic Kidney 293 Cell; SNARE: Soluble N-Ethylmaleimide-Sensitive Factor Attachment Protein Receptor; MAECs: Mouse Aortic Endothelial Cells; CDKN1B: Cell Cycle Protein-Dependent Kinase Inhibitor 1B; CYLD: Cylindromatosis; CRC: Colorectal Cancer; BRG1: Brahma-Related Gene 1; MEF2C: Myocyte Enhancer Factor 2C; ATF2: Activating Transcription Factor2; EC: Esophageal Cancer; APOE: Apolipoprotein E; MMP- 9: Matrix Metalloprotein 9; TH17: T Helper Cells 17; EOC: Epithelial Ovarian Cancer; PEG3: Paternally Expressed Gene 3; ZC3H12B: Zinc-Finger-Type-Containing 12B; PTX: Paclitaxel; TNBC: Triple-Negative Breast Cancer; PSCs: Pancreatic Stellate Cells; PCCs: Pancreatic Cancer Cells; PDAC: Pancreatic Ductal Adenocarcinoma; CTL: Cytotoxic T-Lymphocyte; LNs: Lymph Nodes; DCs: Dendritic Cells; OV: Diolactone
Tumor microenvironment (TME) is a homogeneous population of cells that plays an instrumental role in tumorigenesis, progression, metastasis, chemotherapy and drug resistance [1]. Macrophages are a subset of cells of the immune system that are present in all tissues, including the TME, and are called tumor-associated macrophages (TAMs). Among them, TAMs are the most abundant innate immune cells in the TME, and they play an essential role in the malignant progression of tumors through different mechanisms [2]. It has been reported that TAMs are critical for tumor progression and metastasis and are a major cause of cancer-related mortality [3]. Furthermore, high levels of TAMs infiltration were associated with cancer progression and poorer overall survival in cancer patients [4-6]. This suggests that cellular communication of TAMs significantly affects tumorigenesis, progression, and malignant biological behaviors such as invasion and metastasis. To date, the complex network of exosome-mediated intercellular communication has been widely identified in the TME [7]. As one of the most crucial elements of TME, macrophage-derived exosomes play an indispensable role in mediating intercellular crosstalk at different stages of various cancers [8].
Extracellular vesicles are categorized into three main groups based on size, biological properties and formation process, including exosomes, microvesicles and apoptotic vesicles [9]. Exosomes are extracellular vesicles with a size of 40-150 nm in diameter that are secreted by a diversity of cell types (stromal, stem, immune, tumor, and epithelial cells) under physiological and pathophysiological conditions. Due to the fact that exosomes have properties that reflect those of the parental cells and carry a wide range of different biomolecules, exosomes are thought to be a pivotal factor in mediating cellular communication between tumor cells and microenvironmental cells [10]. In clinical situations, considering that exosomes with favourable biocompatibility can be specifically modified and deliver payloads to target cells, they have been designed to deliver a variety of therapeutic payloads with enormous potential, such as small interfering RNAs, microRNAs (miRNAs/miRs), and small-molecule chemotherapeutic drugs [11]. As a result, targeting exosomal molecules and exosome-based drug delivery systems may revolutionize the current paradigm of conventional cancer treatment regimens. Recently, there has been interest in deciphering the role of macrophage-derived exosomes in cancer. Importantly, macrophage-derived exosomes (MDE), once released into the extracellular environment, are one of the distinct components of the tumor microenvironment and exert their function in the tumor microenvironment [12]. MDE accounts for a relatively large proportion of blood, and high levels of MDE in blood make it a promising biomarker for minimally invasive liquid biopsy, which can be used for the diagnosis and prognosis of cancer patients [13]. For example, proteins in MDE are associated with favorable clinical outcomes in cancer patients. F. Chen et al. identified long chain non-coding RNA (lncRNA) HIF -1α-stabilized long chain non-coding RNA (HISLA) in MDE as a potential biomarker of breast cancer drug resistance [14].
Reports have shown that MDE is a new player in tumor growth, invasion, angiogenesis, inflammatory response, immune remodeling and therapeutic mechanism of action. Furthermore, MDEs can exert a stronger stimulatory immune response to inhibit cancer progression, which means that they have an potential application in antitumor therapy [15]. It is notable that there may be differences between MDEs from different phenotypes. For example, compared to M1-type MDE, M2-type MDE contains higher levels of certain specific miRNAs [16]. These differential miRNAs may contribute to cancer progression and drug resistance, providing new avenues for clinical intervention in cancer patients [17] This suggests that specific exosomes secreted by macrophages may provide novel options for tumor therapy. However, it remains largely unknown which role MDE plays in tumor progression. In this review, we will focus on the mechanism of MDE formation and the vital role it plays in tumor progression. Besides, we also explored the potential application of MDE in targeted therapies, aiming to provide new therapeutic targets, which will help to improve the clinical efficacy of tumor treatments.
Mechanism of Formation for Macrophage-Derived Exosomes
The formation of exosomes and the release of their contents are regulated by a series of precise mechanisms. Exosomes are nanoscale bilayer lipid vesicles secreted by cells and continuously secreted by various types of cells. Macrophage-derived exosomes (MDE) are formed similarly to most cell-derived exosomes in three major stages, including exosome biogenesis, cargo sorting into exosomes, and exosome release [10]. Their biogenesis includes the formation of early and late nuclear endosomes. At first, extramembrane proteins and extracellular components are sequentially formed from plasma membrane invaginations into early endosomes, late endosomes, and multivesicular bodies (MVBs) containing intraluminal vesicles (ILVs). Afterwards, MVBs can either bind to lysosomes or autophagosomes for degradation or be transported to the plasma membrane via the cytoskeleton and microtubule proteins, where they can fuse with the plasma membrane to form exosomes and release them into the extracellular environment [9,18,19]. Typically, most of the mature MVBs are catabolized by lysosomes, and the minority are released into the extracellular environment via extracellular secretion in the form of exosomes with the help of Rab proteins and small GTPasesThese processes are largely mediated by a group of proteins known as the endosomal sorting complex required for translocation (ESCRT), which collaborate with each other throughout the process until exosomes form [20].
Notably, sEVs cargo molecules are essential elements of the biogenesis mechanisms in these processes, including Ras-related proteins Rab GTPases, Syntenin, ESCRT proteins, HSP proteins, four transmembrane proteins (CD9, CD63, CD81, CD82) and lipids [21-24]. Numerous studies have shown that ESCRT-dependent and ESCRT-independent pathways play important roles in the biogenesis of exosomes. The ESCRT-dependent pathway is mainly characterized by the ESCRT complex as a driver of membrane formation and rupture, which is an indispensable regulator of the formation of MVBs and ILVs [25]. The ESCRT protein complex consists of five different complexes, including ESCRT-0, ESCRT-I, ESCRT-II, ESCRT-III, and adenosine triphosphatase-associated vesicle sorting protein 4 (VPS4) complex, which bind to apoptosis-associated gene 2-interacting protein X (ALIX) and vesicle transport 1 (VTA1) proteins to promote the formation and transport of MVBs [26]. Once mature, MVBs can fuse with the plasma membrane to release exosomes into the extracellular space or with lysosomes to degrade their cargoes [27]. An RNA interference study of ESCRT constitutive molecules reveals the role of key ESCRT components in exosome formation and finds that selective inactivation of ESCRT components affects the formation of MVBs and ILVs, which in turn indirectly regulates exosome formation [28]. Mechanistically, autophagy decreases exosome secretion because autophagosomes can fuse with MVBs to form bilayers, which can be catabolized by binding to lysosomes [29,30].
More exocytosis is observed when lysosomes are dysfunctional in macrophages [31]. Conversely, by down-regulating the expression of lysosome-associated membrane protein 1 (LAMP1) and LAMP2, bilaterals in macrophages are unable to bind to lysosomes, which in turn leads to an increased release of exosomes [32,33]. This indicates that MDE is highly dependent on lysosomal function. Significantly, proteins in the ESCRT complex play a dominant role in these processes, and they collaborate with each other throughout the process until exosome formation [34]. ESCRT independent pathways mainly include ceramide and tetraspanin mechanisms. Ceramides are cone lipids whose secretion depends on the action of neutral sphingomyelinase. And once ceramide is generated from sphingolipids, it is readily converted to other bioactive sphingolipids such as sphingomyelin and sphingosine 1-phosphate [35,36]. Trajkovic et al. reported that in oligodendrocytes, inhibition of sphingomyelinase, rather than depletion of ESCRT, significantly reduced exosome formation, a process known as "ceramide-dependent exosome biogenesis" [37]. Further studies suggest that the role played by ceramide in exosome formation may depend on sphingosine 1-phosphate (S1P). S1P is a sphingosine phosphorylation product catalyzed by sphingosine kinase (SphK) ,it binds to the inhibitory G protein (Gi)-coupled S1P receptor,and an essential component of the formation and maturation of MVBs [38]. Besides, some reports have also emphasized that tetratransmembrane proteins such as CD9, CD82, CD63, and membrane proteins of lysosomes/late nuclear endosomes mediate exosome formation [39].
For example, CD63 on the exosome surface is involved in endosomal sorting of melanocytes and regulates exosome formation of ApoE. This mechanism has also been demonstrated in the formation of exosomes of fibroblast origin in patients with Down's syndrome [40]. CD81 protein molecules are cone-like structures with an inner membrane lumen that can accommodate cholesterol. Several conical tetratransmembrane proteins enriched in a cell membrane microdomain can induce ILVs to bud. It was demonstrated that CD9 and CD82 promoted a greater release of exosomes from human embryonic kidney 293 cells (HEK293), which in turn down-regulated β-linker protein expression and inhibited Wnt signaling pathway [41]. Likewise, soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) [42] and GTPase [43] have been recognized as important players in exosome biogenesis and release. Remarkably, Rab proteins further assist in cargo sorting and exosome release. Rab GTPase, the largest of the small GTPase family, regulates several steps in membrane transport, including vesicle outgrowth, vesicle transport along actin and microtubule proteins, and membrane fusion [44]. Moreover, it varies depending on the content and cell type,the biogenesis of MDE is influenced by a variety of extrinsic factors in addition to the biological factors mentioned above. These include cell types, cell status, hypoxia, serum conditions, cytokines and growth factors, drugs and radiotherapy [45-48] 。On the whole, the mechanism of MDE formation is a comprehensive process involving the coordination of a diverse range of molecular cargoes and cellular signaling pathways, mainly including ESCRT-dependent mechanisms, ceramide, and tetratricellular protein mechanisms. Strikingly, it is worth noting that an increase or decrease in MDE release has different effects on different diseases.
Macrophage-Derived Exosomes are Involved in Tumor Progression
It is crucial that bidirectional interactions between cancer cells and TAMs are largely mediated by bioactive molecules delivered by exosomes. Remarkably, the role of MDE in tumorigenesis and development is complex and multifaceted. It is generally recognized that tumor cells secrete a variety of exosomes containing several types of molecules and transport these molecules through the blood or surrounding cells in the TME [49]. In contrast to cytokines secreted directly by macrophages, these mediators are also contained in the MDE and can be transported to recipient tumor cells, thereby promoting tumor biological functions such as proliferation, invasion, and vascularization, and significantly affecting tumor progression and metastasis (Figure 1). Furthermore, these molecules may also contribute to the immunosuppressive microenvironment by regulating immune cells. The most prominent molecules contained in MDE are various types of RNA molecules,,MicroRNAs appear to be the most abundant regulatory RNAs in exosomes [7]. A growing number of studies in recent years have elaborated on the effects of MDE on cancer behavior. For instance, MDE has been found to promote cancer progression and cellular drug resistance in osteosarcoma by activating the AKT pathway [50]. In another study, M1 macrophage-derived exosomes (M1-exos) with high levels of miR-326 down-regulated NF-κB expression in hepatocellular carcinoma (HCC) via miR-326, which in turn inhibited HCC cell proliferation, migration, and invasion, and promoted apoptosis in HCC cells [51]. MiR-501-3p in M2 macrophage-derived exosomes (M2-exos) promoted tumor development by activating the transforming growth factor-β signaling pathway and suppressing the tumor suppressor gene TGFBR [52]. Similarly, miR-let-7a-5p in MDE can be transferred to lung cancer cells by down-regulating Bcl2-like 1 (BCL2L1) expression, inhibiting cell proliferation, migration and invasion [53]. Taken together, these results support a crucial role for MDE in mediating cancer progression. In the following, we will highlight the role that MDE plays in cancer progression (Table 1) [54-67].
Figure 1 Exosomes derived from macrophages have potential role in promoting tumor growth, invasion and metastasis, modulating immune escape and drug resistance.
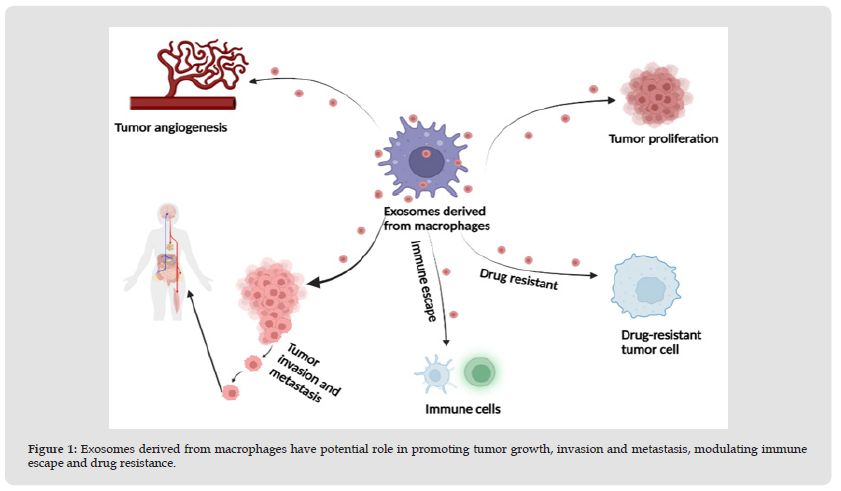
Macrophage-Derived Exosomes Regulate Tumor Angiogenesis and Proliferation
Tumor growth and metastasis are heavily dependent on angiogenesis, the process of growing new blood vessels from existing vessels surrounded by a growing tumor mass. Tumor angiogenesis involves multiple processes and cell types [68]. MDE has been shown to promote angiogenesis by shaping the immune microenvironment [69]. An increasing number of studies have recently evaluated the role of MDE in tumor angiogenesis. MDE has been reported to accelerate angiogenesis by carrying various cargo molecules. For example, M2-exo has been shown to promote angiogenesis in mouse aortic endothelial cells (MAECs) both in vivo and ex vivo. Additionally, the researchers analyzed by RNA sequencing and qPCR that miR-155-5p and miR-221-5p are contained in M2-exo, which can be transferred to MAECs [70]. Similarly, miR-501-3p in M2-exo enhanced the tube-forming ability of pancreatic ductal adenocarcinoma (PDAC) cells by increasing the expression levels of angiogenesis-related proteins (VEGFA, VEGFR2, ANG2, and PIGF), suggesting that miR-501-3p is essential for angiogenesis in PDAC [52]. Another study reported that miR-155-5p and miR-221-5p in M2-exo promoted tumor growth and angiogenesis in PDAC by targeting E2F2 [70]. E2F2, a member of the E2F family of transcription factors, plays an important role in the regulation of cell cycle progression, and loss of E2F2 expression accelerates tumor growth and metastasis [71].
On the other hand, MDE significantly reduces the secretion of pro-inflammatory cytokines and stimulates the proliferation and migration of endothelial cells, thus improving angiogenesis and re-epithelialization in diabetic wounds [72]. Overall, these results confirm the interaction of MDE with cancer angiogenesis, and targeting these pro-angiogenic exosomes may provide new avenues for solid tumor treatment considering the clinical application of anti-angiogenic therapies. The role of M2-Exo in the tumor microenvironment is similar to that of M2 macrophages. For one thing, M2-Exos can promote the proliferation of tumor cells. Cell cycle protein-dependent kinase inhibitor 1B (CDKN1B) has been recognized as an inhibitor of cell cycle progression during the G1/S transition. Low levels of CDKN1B have been associated with tumor cell proliferation and poor prognosis [73]. miR-221-3p in M2-Exos promoted tumor cell proliferation and G1/S transformation by decreasing CDKN1B levels in tumor cells [58]. Other studies have shown that miR-588 in M2-Exos contributes to enhancement of gastric cancer (GC) cell proliferation and inhibition of apoptosis by targeting cylindromatosis (CYLD) [74]. Based on these studies, M2 -Exos is an important factor in accelerating cancer cell proliferation, which may be associated with precancerous lesions and suppression of tumor suppressor genes. Collectively, M2-Exos plays a crucial role in both tumor angiogenesis and proliferation.
Macrophage-Derived Exosomes Regulate Tumor Invasion and Metastasis
MDE promotes specific tumor biological behaviors by inducing recipient cells to acquire specific biological phenotypes. Tumor metastasis is a multistep process in which tumor cells leave the primary site, enter the circulation, and attach to vascular endothelial cells, forming a key link in the metastatic process [75]. Therefore, we should further explore the role of MDE throughout each step from primary tumor cell development to metastasis formation. There is growing evidence indicating that MDE plays an influential role in promoting the rate of cancer initiation and progression. In a mouse model, miR-21-5p and miR-155-5p were significantly up-regulated in M2-Exos and miR-21-5p was taken up by colorectal cancer (CRC) cells and bound to the Brahma-related gene 1 (BRG1) coding sequence to down-regulate the expression of BRG1, which promotes CRC cell migration and invasion [56]. Similarly, M. Yang et al. reported that miR-223 from MDE can be delivered to breast cancer cells to inhibit the expression of myocyte enhancer factor 2c (Mef2c), which in turn leads to invasion and metastasis of breast cancer cells [76]. On the one hand, M2-Exos can down-regulate miR-26a through high expression of lncRNA AFAP1-AS1 and activating transcription factor2 (ATF2) expression, which in turn promotes esophageal cancer (EC) cell migration and invasion [70]. On the other hand, LncRNA LIFR-AS1 was highly expressed in MDE and contributed to osteosarcoma cells proliferation and invasion through the miR-29a/NFIA axis [76]. Apolipoprotein E (ApoE) is a highly specific protein in M2 -Exos. As soon as gastric cancer cells took up ApoE in M2 -Exos, the PI3K/AKT signaling pathway was activated to promote gastric cancer cell migration. Notably, ApoE-/- mice M2 -Exos did not affect gastric cancer cell migration [77].
Integrin αMβ2 (CD11b/CD18) was also recognized in M2-Exos with remarkable specificity and efficiency. Exosome-mediated transfer of CD11b/CD18 proteins from macrophages to hepatocytes can further activate the matrix metalloprotein 9 (MMP-9) signaling pathway and enhance the migration of hepatocellular carcinoma cells [78]. In another study, miR-29a-3p and miR-21-5p in M2-Exos promoted cancer progression and metastasis by enhancing the ratio of T regulatory cells (Treg) to T helper cells 17 (Th17) to establish a cancer immunosuppressive microenvironment [17]. Dramatically, MDE can also inhibit tumor progression. For example, Y. Hu et al. showed that after miR-7 in MDE was transferred to epithelial ovarian cancer (EOC) cells, miR-7 inhibited EOC cell metastasis by decreasing the activity of the EGFR/ AKT/ERK1/2 pathway [54]. As well, miR-223 in MDE promotes GC cell migration and invasion through the PTEN-PI3K/AKT pathway [79]. In medulloblastoma, miR-155-3p in M2-Exos plays a role in tumor cell invasion and migration [80]. Moreover, miR-21-5p and miR-155-5p were highly enriched in M2-Exos, which down-regulated the protein expression of BRG1 in CRC cells and promoted cancer cell migration and invasion [81]. In conclusion, these data suggest that MDE-metastatic cargo molecules not only target cancer cells directly, but also have an indirect effect on cancer cells through immune cells. Accordingly, MDE may provide new targets for cancer therapy. It is worth noting that the involvement of MDE in other metastatic steps remains to be further explored. Examples include the effect of MDE on the entry and exit of tumor cells from dormancy, and communication between CTCs or clusters of circulating tumor cells and other circulating components (monocytes, NK cells, neutrophils, and platelets).
Macrophage-Derived Exosomes Regulate Tumor Cell Immune Evasion
Several studies have shown that MDE can reduce the attack of immune cells on tumor cells by altering the properties of tumor cells, thus promoting the escape of tumor cells from immune recognition. It has been reported that MDE from TME modulates the immune response. For example, miR-21 in MDE facilitates glioma cell proliferation, migration and invasion. More importantly, miR-21 promotes glioma cell immune escape by inhibiting the expression of paternally expressed gene 3 (PEG3), leading to decreased CD8+ T cell proliferation, decreased cellular activity, and decreased IFN-γ levels [60]. Furthermore, CCL5 in MDE promoted CRC cell immune escape through the p65/STAT3-CSN5-PD-L1 signaling pathway [82]. In epithelial ovarian cancer (EOC), miR-29a-3p and miR-21-5p in MDE upregulate the regulatory T cell (Treg)/T helper (Th)17 cell ratio, which in turn promotes immune evasion [17]. M1-Exos can carry miR-181a-5p to target ETS1 and inhibit STK16 expression in tumor cells, thereby reducing cell viability and promoting apoptosis in lung adenocarcinoma cells [83]. It should be noted that M1-Exo can inhibit tumor progression by directly promoting apoptosis or enhancing tumor immune response. In addition, M1-Exos can carry miR-16-5p to reduce the expression of PD-L1 in gastric cancer cells, thus reducing the inhibitory effect of immune checkpoints on T cells and promoting the activation and killing ability of T cells [84]. C. Cianciaruso et al. collected MDE in mice and quantified them, and through proteomic and lipidomic analyses, they found that MDE in mice showed a molecular profile associated with Th1 / M1 polarization, enhanced inflammatory and immune responses, and a more favorable patient prognosis. Importantly, MDE also promoted T cell proliferation and activation [85].
It is interesting to note that H. Jiang et al. found that M1-Exos could act as a tumor suppressor, and that M1-Exos highly expresses lncRNA HOTTIP, which competitively binds miR-19a-3p and miR-19b-3p to up-regulate the TLR5/NF-κB signaling pathway, thereby inhibiting the proliferation, invasion, and metastasis of head and neck squamous cell carcinoma cells [86]. Furthermore, F. Zhang et al. showed that the lncRNA AGAP2 antisense RNA 1 (AGAP2- AS1) in M1-Exos enhances lung cancer radiotherapy immunity by decreasing miR-296 and increasing notch homolog protein 2 (NOTCH2) [87]. Evidence suggests that miR-155-5p in M2-Exos may promote immune escape from colon cancer by inhibiting Zinc-finger-type-containing 12B (ZC3H12B) expression, which results in decreased IL-6 stability, thereby promoting colon cancer incidence and progression [88]. The same way, in hepatocellular carcinoma, miR-21-5p in M2-Exos promotes CD8+ T cell depletion and reduces the killing capacity of CD8+ T cells through the YOD1/YAP/β-catenin pathway [89]. It is interesting to observe that miR-29a-3p in M2-Exos was able to down-regulate the FOXO3-AKT/GSK3β axis, promote PD-L1 expression on the surface of tumor cells, and ultimately promote the binding of PD-L1 and PD-1 on the surface of CD8+ T cells, inhibit their killing function, and lead to immune escape [90] Moreover, in addition to affecting CD8+ T cells, it also regulates the ratio of two subpopulations of CD17 T cells, Tregs and Th4 cells. Tregs suppress tumor immunity, whereas Th17 cells regulate the activation of cytotoxic CD8+ T cells. miR-29a-3p and miR-21-5p in M2-Exos were transferred to CD4+ T cells, where they were able to directly inhibit STAT3, inducing an imbalance in the Treg/Th17 cell ratio and generating an immunosuppressive microenvironment that promotes the progression and metastasis of epithelial ovarian cancer [17]. These studies demonstrated that MDE can suppress local immunity and promote tumor immune escape and progression, which may represent a potential target for cancer immunotherapy. However, MDE-induced immunomodulation is complex and dynamic, and its related mechanisms remain to be further elucidated.
Macrophage-Derived Exosomes Modulate Drug Resistance in Tumor Cells
Current research has identified drug resistance as another barrier to clinical efficacy. Cisplatin is a widely used platinum-based compound with clinical activity against a variety of solid tumors [91]. A new study revealed that miR-21 and miR-155 in MDE contribute to cisplatin resistance in gastric cancer cells and adult neuronal cell tumor cells, respectively [92,93]. Gemcitabine is to be considered an essential cytidine analog with potent antitumor effects against a wide range of tumors [94]. Y. Binenbaum et al. showed that miR-365 in MDE was translocated into pancreatic ductal adenocarcinoma cells and induced gemcitabine resistance by up-regulating cytidine deaminase levels, whereas immunotransfusion of miR-365 antagonists restored sensitivity to gemcitabine [16]. Interestingly, miR-223 in MDE enhanced drug resistance in EOC cells via the PTEN-PI3K/AKT pathway both in vivo and ex vivo when EOC were under hypoxic conditions [55]. Recent studies have reported that M2-Exos can increase chemotherapy resistance in tumor cells. It has been shown that miR-223 in M2-Exos increases resistance to adriamycin and oxaliplatin by inhibiting the F box and WD repeat-containing structural domain-7 (FBXW7) pathway in gastric cancer cells [95]. Furthermore, decreased lncRNA-CRNDE expression in M2-Exos was associated with chemoresistance in gastric cancer, while its increase showed the opposite effect. A recent study showed that high levels of exon-lncRNA-CRNDE enhanced cell proliferation and tumor progression by targeting PTEN expression, and that inhibition of CRNDE in M2-Exos enhanced sensitivity to cisplatin in gastric cancers [63].
Thereby, targeting exosomal miR-21 from TAM may be a promising target for cisplatin-resistant patients with gastric cancer. These studies suggest that MDE may be involved in a feedback loop between cancer cells that enhances tumor resistance. Consequently, cargo molecules in MDE may be a promising new therapeutic target for chemoresistance in cancer patients. However, the potential mechanism by which MDE causes chemoresistance in cancer cells remains unclear. In conclusion, there is a growing interest in further understanding the mechanisms of MDE-induced cancer progression, drug resistance and their potential clinical applications. ND, not determined; EOC, epithelial ovarian cancer; OC, ovarian cancer ; BC, breast cancer; HCC, hepatocellular cancer; PDAC, pancreatic ductal adenocarcinoma; CRC, colorectal cancer; GC, gastric cancer; NBL, neuroblastoma; LC, lung cancer; GBMLGG, glioma; PCa, prostate cancer; BC, bladder cancer; OS, osteosarcoma; EC, esophageal cancer; PTEN, phosphatase and tensin homolog; PI3K, phosphoinositid-3-kinase; AKT, protein kinase B; EGFR, epidermal growth factor receptor; CDKN1B, cyclin-dependent kinase inhibitor 1B; STAT3, transcription 3; IGF-1R, insulin-like growth factor-1 receptor; NTP, triphoshphate-nucleotide; BRG1, brahma-related gene 1; BCL2L1, Bcl2-like 1; PEG3, paternally expressed gene 3;; NEDD4-1, protein 4‐1;PTEN, phosphatase and tensin homolog; NFIA, nuclear factor I/A; XIAP, X‐linked inhibitor of apoptosis protein; ATF2, activating transcription factor 2.
Macrophage-Derived Exosomes for Cancer Therapy
Since MDE can transfer cargo to receptor cells and has the intrinsic ability to cross natural barriers in the body, it is able to specifically deliver its payload to hard-to-reach sites [96]. With good biocompatibility, low toxicity, low immunogenicity, wider distribution and chemical stability of exosomes compared to other nanocarriers, as well as their potential ability to induce activation/modification of multiple pathways in receptor cells as natural carriers, the potential use of exosomes as nanomedicine has gained increasing attention in the scientific community [97,98]. For instance, it has been shown that due to the presence of CD47 on the surface of exosomes, phagocytosis by circulating monocytes can be efficiently avoided, thus promoting the metastasis of cargo. Therefore, using exosomes as carriers and modifying them for clinical applications by artificially optimizing the integration of specific loads such as tumor drugs and targeted siRNAs is a promising and inspiring idea [99,100]. This denotes that exosome-based therapies are emerging cutting-edge strategies to inhibit tumor progression. Moreover, considering their natural products and their role in different stages of cancer development, they may provide an effective anti-cancer immune [101].
M1-exos not only acted as a drug carrier to deliver paclitaxel to tumors to enhance the delivery of anticancer drugs, but also enhanced the antitumor effect by increasing the activation of caspase-3-induced apoptosis and the production of proinflammatory cytokines [102]. Recently, M.J. Haney and coworkers exploited the natural convergence of MDE to the cancer microenvironment in order to improve paclitaxel (PTX) and adriamycin (DOX) delivery in triple-negative breast cancer (TNBC) [103]. In Lewis lung cancer in mice, MDE has a targeting ability for lung cancer metastasis. Among them, injection of exosomes loaded with paclitaxel significantly inhibited metastatic growth compared with paclitaxel [104]. M.S. Kim et al. developed and optimized MDE loading with paclitaxel, and they demonstrated that engineered MDE treated with the chemical agent paclitaxel enhanced the antitumor efficacy of lung metastases in mice [105] A study showed that MDE delivers brain-derived neurotrophic factors by overcoming the blood-brain barrier to ameliorate central nervous system disorders, which in turn removes residual nanomaterials to mitigate toxicity and generation in brain tumor therapy [106,107]. Y. Zhao et al. recently isolated exosomes from macrophages and developed a specific MDE-based anti-pancreatic cancer Gemcitabine (GEM)-resistant drug delivery system. A nanoplatform for pancreatic cancer therapy [108].
Meanwhile, MDE loaded with cisplatin enhances its anticancer effects by inhibiting the proliferation of cancer cells, inducing their apoptosis and increasing their drug sensitivity [109,110]. S. Rayamajhi et al. combined macrophage-derived exosomes with synthetic liposomes and the bionic exosome, which not only increased exosome production but also improved tumor site targeting through adriamycin encapsulation [111]. Previously, exosomes derived from pancreatic cancer cells (PCCs), pancreatic stellate cells (PSCs) and macrophages were used to deliver adriamycin. It was found that of the three types of exosomes, those of PCCs origin had the highest drug-carrying efficiency, whereas macrophage-derived exosomes carried adriamycin with the highest antitumor effect [112]. As a result, targeting MDE would be a potential strategy for the treatment of tumors. Importantly, MDE also has the potential to reduce adverse effects in drug therapy [112]. Due to the presence of cargo molecules, MDE exhibits good tumor targeting and deep tumor penetration. Recent studies have confirmed that MDEs have great potential in treating diseases by acting as delivery vehicles for genes and proteins. They can modify parental cells and use transgenes to cause these cells to secrete therapeutic proteins containing desired [113].
In a study by Y. Binenbaum et al, artificial dsDNA (barcode fragment) was transfected into mouse peritoneal macrophages and injected into mice bearing Pancreatic ductal adenocarcinoma (PDAC) tumors. The results showed that the concentration of barcode fragments in primary tumors and liver metastases was 4-fold higher than in normal tissues, an effect mediated by the transfer of miR-365 in MDE. miR-365 was detected by upregulation of the triphospho-nucleotide pool in cancer cells and the induction of the enzyme cytidine deaminase to impair gemcitabine activation; the latter causes the loss of gemcitabine [16]. MDE also enhances the activity of anti-tumor vaccines and promotes cytotoxic T-lymphocyte (CTL)-associated immune responses, thereby significantly impeding tumor progression. Specifically, M1-exos can be taken up by local macrophages and dendritic cells (DCs) in lymph nodes (LNs), leading to increased Th1 cytokine release and enhanced CTL responses [15]. Detailed findings further suggest that exosomes shed by macrophages are a potent immune adjuvant in cancer immunotherapy and lead to tumor regression in fibrosarcoma [114]. Targeting the biogenesis and constituents of MDE may have substantial clinical applications in antitumor therapy [115]. To illustrate, A.T.H. Wu et al. showed that follicular diolactone (OV) reduced miR-21 enrichment in M2-exos, which in turn inhibited bladder carcinogenesis [56]. To summarize, MDEs have multiple advantages in anticancer therapy due to their excellent tumor-targeting ability. In particular, they can act as drug carriers and can even cross the blood-brain barrier, leading to a potential targeting of personalized nanomedicines capable of delivering tremendous anticancer effects. Notably, effective technologies still need to determine the appropriate drug-carrying capacity to further increase the targeting effect of exosomes and reduce cytotoxicity.
In summary, MDE plays a complex and significant role in tumor progression. An in-depth understanding of the formation mechanism of MDE, its interaction with tumor cells, and its potential in tumor therapy provides strong support for the development of new strategies and targets for cancer immunotherapy. When MDEs are used as carriers, they bind to receptors on target cells to deliver loaded drugs such as proteins and nucleic acids. In addition, MDE function is influenced by macrophage polarization. Meanwhile, MDEs are being designed as delivery systems, which could dramatically change the current status quo of cancer treatment regimens. In this review, we have investigated the formation mechanisms of MDE biogenesis. In addition, we also studied the functional properties of MDE in tumor angiogenesis, growth, invasion and metastasis, immune evasion, and drug resistance.MDE provides multiple pathways for tumor therapy by carrying targets, acting as a delivery agent, and mediating paracrine effects. Although many RNAs, proteins and lipids have been identified in MDE, their functions remain unclear. Further studies will help to reveal the biological functions and regulatory mechanisms of MDE and provide new ideas and breakthrough points for the clinical application of MDE as a cancer therapeutic tool.
Nonetheless, MDE-mediated functions need to be explored more. For one thing, the biological mechanisms that guide specific molecules into exosomes are still not fully understood, and more research is needed to explore the underlying mechanisms and how they are altered in different environments and stages. The yield of MDEs remains low, moreover, and standardized methods for extracting and purifying exosomes for clinically relevant applications have not yet been established, which limits the potential use of MDEs as diagnostic and prognostic markers, as well as their application as targeted drug carrier systems. Finally, although engineered MDEs significantly improve targeting, their safety should be evaluated. Although the oncogenic components of exosomes have been removed in studies, there is still a potential risk in clinical applications because exosomes are isolated from cancer cells. Gradually conducting relevant clinical trials may be a worthwhile goal once the safety of this treatment is clarified. We can reduce the production of these cancer-causing exosomes or reduce the expression of cancer-causing components in the exosomes through genetic modification and improve the prognosis of patients by combining immunotherapy and radiotherapy. It is believed that with the development of biotechnology, MDE will play a pivotal role in the diagnosis, prevention and treatment of diseases in the future. In the meantime, we need to explore whether MDE can function independently of cells.
Jing Zhang involved in edited the manuscript, Xiaomei Yi searched for literature, Jianwei Wu wrote the manuscript and supervised the project and contributed to the revision of the final manuscript. All authors contributed to the article and approved the submitted version.
We deeply appreciate the support from all participants. The images in this article were created by BioRender.com.
This research was funded by Science and Technology Program of Health Commission of Jiangxi Province (SKJP220227588)
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.


