Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Abu OD1*, Ojo I2 and Awhin EP3
Received: August 08, 2023; Published: August 18, 2023
*Corresponding author: Abu OD, Department of Biochemistry, Faculty of Life Sciences, University of Benin, Benin City, Nigeria
DOI: 10.26717/BJSTR.2023.52.008233
The present study investigated the protective property of ethanol extract of Cucumis sativus on streptozotocin (STZ)-induced diabetic rat pancreas. Male albino rats of Wistar strain (n = 25, mean weight = 215 ± 15 g) were assigned to five groups (5 rats/group): normal control, diabetic control, metformin, extract (200 mg/ kg body weight, bwt) and extract (300 mg/kg bwt) groups. A single intraperitoneal injection of 50 mg/kg bwt STZ was used to induce diabetes mellitus in the experimental rats. The diabetic rats were treated for a period of 21 days with 50 mg/kg bwt metformin (standard antidiabetic agent) or the extract. Activities of catalase, superoxide dismutase (SOD), glutathione peroxidase (GPx) and glutathione reductase (GR) as well as glutathione (GSH), total protein (TP), malondialdehyde (MDA) and nitric oxide (NO) levels were measured in pancreatic tissue. The results showed that the diabetogenic agent STZ significantly increased the fasting blood glucose (FBG) concentrations of the rats, but it decreased the activity/concentration of antioxidant enzymes/molecules (p < 0.05). However, treatment of the diabetic Wistar albino rats with 200 and 300 mg/kg bwt extract markedly reduced the FBG concentration and body weights of rats, but enhanced the activity/concentration of antioxidant enzymes/molecules in pancreatic tissue (p < 0.05). These results indicate that ethanol extract of C. sativus fruit has the potential to promote antioxidant defense in STZ-induced diabetic rats pancreas.
Keywords: Cucumis Sativus; Glutathione; Lipid Peroxidation; Medicinal Plant; Oxidative Stress
Abbreviations: SOD: Superoxide Dismutase; GPx: Glutathione Peroxidase; GR: Glutathione Reductase; GSH: Glutathione; TP: Total Protein; MDA: Malondialdehyde; NO: Nitric Oxide; FBG: Fasting Blood Glucose; ROS: Reactive Oxygen Species; AP: Acute Pancreatitis; O-GlcNAcase: O-linked β -N-Acetylglucosaminase
Pancreas, a glandular organ in the digestive and endocrine systems of vertebrates, is located in the abdominal cavity behind the stomach. As an endocrine gland it synthesizes insulin, glucagon, somatostatin, and pancreatic polypeptide [1]. The pancreas is also a digestive organ, secreting pancreatic juice which contains enzymes that facilitate digestion and absorption of nutrients in the ileum. Anatomically, the pancreas is divided into a head, which rests within the concavity of the duodenum, a body lying behind the base of the stomach, and a tail, which ends abutting the spleen. Lying anterior to the superior mesenteric artery and vein, the neck of the pancreas resides between the body and head. The head of the pancreas surrounds these two vessels, and a small uncinate process emerges from its lower part, lying behind the superior mesenteric artery [1]. The pancreas has an internal hormonal role (endocrine) and an external digestive role (exocrine). It has two major ducts, the main pancreatic duct, and the accessory pancreatic duct [2]. The functional state of the pancreas is evaluated via determination of the activities of α-amylase, lipase and elastase (in stool) [2]. Certain drugs have been demonstrated to cause acute pancreatitis [3,4]. The role of oxidative stress in disease pathogenesis is well documented. Oxidative stress-triggered mitochondrion membrane rupture leads to pancreatic cell necrosis.
Reactive oxygen species (ROS) produced within the cells rupture lysosomal membrane releasing hydrolases [3,5]. Pancreatotoxicity is characterized by widespread alterations of membranous organelles of acinar cells, especially the endoplasmic reticulum and zymogen granules. Hemorrhagic necrosis of the pancreas is due to endogenous, intraparenchymal activation of zymogen proteases, including proelastase [5]. The necrotic event may also be characterized by severe congestion and edema of the stroma, which contains large quantities of extravasated red and inflammatory cells (mostly neutrophils).
Diabetes mellitus is generally induced in laboratory animals by several methods: chemical, surgical and genetic (immunological) manipulations. Chemical method include the use of STZ, alloxan and sucrose load. Synthesized by a strain of the soil microbe Streptomyces achromogenes (Gram-positive bacterium) STZ is a permanent diabetes inducing drug [4]. Induction of diabetes mellitus with STZ involves damage to the pancreas. This study investigated the protective property of ethanol extract of C. sativus on STZ-induced diabetic rat pancreas.
All chemicals and reagents used in this study were of analytical grade and they were products of Sigma-Aldrich Ltd. (USA).
Freshly harvested C. sativus fruits were purchased from a major vegetable market in Benin City, Nigeria and identified at the Department of Plant Biology and Biotechnology, University of Benin, Benin City, Nigeria. They were washed and air-dried for 4 weeks at the Department of Biochemistry. The dry plant was ground with a mechanical blender. The pulverized sample was cold macerated in absolute ethanol for three days (72 h) in a bell jar and filtered using Whatmann filter paper No. 42 (125 mm). The ethanol extract was thereafter concentrated using rotary evaporator and freeze-dried using a lyophilizer [6-10].
Mature male Wistar albino rats (n = 25, mean weight = 215 ± 15 g) were obtained from the Department of Anatomy, University of Benin, Nigeria and housed in wooden cages. They were acclimatized for two weeks before commencement of the study and had free access to feed and clean water.
The rats were assigned randomly to five groups (5 rats/group): normal control, diabetic control, metformin, extract (200 mg/kg bwt) and extract (300 mg/kg bwt) groups. A single intraperitoneal injection of 50 mg/kg bwt STZ was used to induce diabetes mellitus in the experimental rats. Subsequently, the diabetic rats were treated for a period of 21 days with either metformin (50 mg/kg bwt) or the medicinal plant extract.
At the end of 21-day treatment, the rats were euthanized under mild chloroform anaesthesia (after an overnight fast). Their pancreases were excised, blotted dry and used to prepare 20 % tissue homogenate. The homogenate was centrifuged at 2000 rpm for 15 min to obtain clear supernatant which was used for biochemical analysis. The activities of catalase, SOD, GPx and α-amylase were determined [11-14]. Levels of pancreatic TP, MDA, GSH, and NO were also measured [15-18]. The activity of GR was determined using a previously described method [19].
Data are presented as mean ± SEM (n = 5). Statistical analysis was performed using SPSS version 21. Statistical differences between means were compared using Duncan multiple range test. Statistical significance was assumed at p < 0.05.
As shown in (Tables 1 & 2), STZ-induced diabetes mellitus significantly increased the blood glucose concentration of the rats (p < 0.05). However, treatment of the diabetic rats with extract of C. sativus fruit markedly reduced their FBG concentration and body weights (p < 0.05).
Table 1: Effect of Ethanol Extract of C. sativus on Weight and Blood Glucose Parameters.

Note: Data are weight and FBG parameters and are expressed as mean ± standard error of mean (SEM, n = 5).
Table 2: Comparison of Organ and Relative Organ Weights.
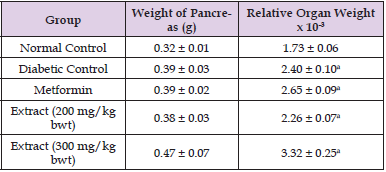
Note: Data are organ/body weight ratio of rat and are expressed as mean ± SEM (n = 5).
Values with superscript “a” are significantly different from the normal control group.
Treatment of STZ-induced diabetic Wistar rats with ethanol extract of C. sativus significantly increased the activities of the antioxidant enzymes as well as concentrations of GSH and NO, but it markedly reduced the activity of α-amylase and concentrations of pancreatic TP and MDA (p < 0.05). These results are shown in (Tables 3-6).
Table 3: Effect of Ethanol Extract of C. sativus on Pancreatic Total Protein and Glutathione Levels.

Note: Data are pancreatic total protein and GSH levels and are expressed as mean ± SEM (n = 5).
Values with superscript “a” are significantly different from the normal control group.
Values with superscript “b” are significantly different from the diabetic control group.
Table 4: Effect of Ethanol Extract of C. sativus on Oxidative Status in Rat Pancreas.

Note: Progressive motility (PM); Linearity (LIN); Straightness (STR); Trail speed (VCL); Progressive Speed (VSL); Speed of travel (VAP); Amplitude of lateral head displacement (ALH); Total Motility (MT); wobble index (WOB); Micromeres per second (μm/s).
Table 5: Effect of Ethanol Extract of C. sativus on Pancreatic Alpha- Amylase and Glutathione Peroxidase Activity.

Note: Data are activities of pancreatic α-amylase and glutathione peroxidase and are expressed as mean ± SEM (n = 5).
Values with superscript “a” are significantly different from the normal control group.
Values with superscript “b” are significantly different from the diabetic control group.
Table 6: Effect of Ethanol Extract of C. sativus on Pancreatic NO Level.

Note: Data are pancreatic NO concentrations and are expressed as mean ± SEM (n = 5).
Acute pancreatitis (AP) may be caused by exposure to certain drugs/compounds [3] Literature has shown that about 20 % of the top most prescribed drugs are toxic to the pancreas [4,5]. Some causes of pancreatitis are gallstones, alcohol abuse, hypercalcemia, hypertriglyceridemia, viral infection, trauma as well as cardiovascular and anti-inflammatory analgesic agents [20]. Pancreatotoxicity due to direct exposure to drugs results in subclinical pancreatic damage [21]. Two out of the following three features are required for a diagnosis of AP:
a) Abdominal pain characteristic of acute pancreatitis;
b) Serum amylase and/or lipase levels ≥ 3 times the upper limit of normal; and
c) Results of CT scans [6].
As a localized inflammation of the pancreas the disease is commonly mediated by the premature activation of digestive enzymes retained in the organ. Although this condition may resolve by itself within days, its persistence could cause pancreatic dysfunction and failure of other remote organs/systems [22]. Pancreatitis is of two forms: acute and chronic. It was recently discovered that chronic pancreatitis is a consequence of repeated episodes of an acute case [23]. One of many chemical agents that can damage the pancreas is STZ. Induction of diabetes mellitus with STZ causes serious damage to the organ [11]. The exact molecular mechanism underlying the cytotoxic effect of STZ is not well-understood, however studies suggest that the cytotoxicity could be via production of reactive oxygen species (ROS) thus inducing oxidative stress, causing DNA damage with resultant necrosis (due to the DNA methylating activity of the methyl nitroso urea moiety of the drug), release of NO which inhibits aconitase activity resulting in mitochondrial dysfunction, or via inhibition of O-linked β -N-acetylglucosaminase (O-GlcNAcase) [24]. Increasing evidence suggest that oxidative stress plays a role in the pathogenesis of diabetes mellitus and its complications [25]. Hyperglycemia increases oxidative stress, which contributes to impairment of processes that fail during diabetes (insulin action and secretion). In addition, antioxidant mechanisms are diminished in diabetic patients, which may further promotes oxidative stress [26,27].
This study investigated the protective property of ethanol extract of C. sativus on STZ-induced diabetic rat pancreas. The results showed that induction of diabetes mellitus with STZ significantly increased the FBG concentrations of the rats, but it decreased the activity/concentration of antioxidant enzymes/molecules. However, treatment of the diabetic Wistar albino rats with two different doses of ethanol extract of C. sativus fruit markedly reduced the FBG concentration and body weights of rats, but enhanced the activity/concentration of antioxidant enzymes/molecules in pancreatic tissue. These results are in agreement with those of previous studies [28-33]. The results suggest that oxidative stress plays a crucial role in STZ-induced diabetes mellitus and that increased ROS production suppresses the activity of antioxidant enzymes, while diminishing the levels of antioxidant molecules in pancreatic tissue. The beneficial effect of the medicinal plant may not be unconnected to its bioactive compounds. Studies have shown that plants containing important phytochemicals have many pharmacological/biological effects [34-67].
The results obtained in this study indicate that ethanol extract of the medicinal plant fruit has the potential to promote antioxidant defense in STZ-induced diabetic rat pancreas.


