Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Moges Abebe*
Received: December 22, 2022; Published: January 20, 2023
*Corresponding author: Moges Abebe, Department of Biological and Physical Sciences, Saint Augustine University, USA
DOI: 10.26717/BJSTR.2023.48.007617
In this solvent selection experiment, liquid-solid extraction was utilized to separate dried Moringa oleifera leaves using a binary solvent mixture of ethanol and water. This process of using 0–100% ethanol in water worked as a binary solvent for both polar and nonpolar solutes. A negligible amount of reaction occurred as the two miscible solvents were mixed, as they are both highly polar and have similar pKa values. A chemical extraction experiment was performed, Results indicated that the 60–65% ethanol-water combination is a better solvent for the extraction. Mover ever, using this method, the following several medicinal compounds were identified: fiber and polysaccharide products (75.49%), fats and waxes (3.085%), phenolics and terpenoids (12.975%), and alkaloids (8.450%). All these results indicate that the dried leaves have a large quantity of fiber and many vitamins and nutrients embedded in the plant.
Moringa leaves have shown promising bioactivity, including antibacterial, antihypertensive, and anti-inflammatory properties [1]. There are many reports in science that moringa has a glucose-lowering effect, cholesterol-lowering in plasma and liver, anti-obesity properties, cardioprotection, and a reduction in insulin resistance. Moringa leaves are high in flavonoids, isothiocyanates, phenolic acids, saponins, tannins, and vitamins, which are mostly found in the plant’s leaves [2]. In the present study, moringa leaves were selected because they are considered by many to be superfoods and have medicinal uses. They provide a substantial source of bioactive compounds that make them a rich source of nutrition as well as having disease-fighting properties. To make moringa, which is known as a miracle tree full of vitamins and nutrients, less toxic ethanol and water were selected as the binary solvents for extraction. The roots, stems, and leaves are consumed as superfoods despite the fact that the roots and bark are toxic and only the leaves are recommended for daily consumption [3]. The fiber and antioxidative properties of the plant cause long-term erosion of the stomach, intestine, and the collapse of the kidneys, the liver, and other organs in the intestinal tract. Given that moringa contains over 83 different types of bioactive compounds, consuming a large amount of the plant is required to obtain comparable nutritional values of the nutrients. Extracts from moringa leaves are not medicine by themselves, but they are supportive of the disease when taken with other foods and taken the right way. Furthermore, pregnant women are discouraged from consuming moringa, as it hurts the unborn fetus in the womb. The solvent used to extract the organic and inorganic compounds was carefully selected as it will have a major impact on human consumption.
Ethanol is an organic solvent that is safe and economical and has anti-bacterial and anti-virus properties. Ethanol is a flammable, colorless, grain alcohol with the ability to mix miscibly with many polar and nonpolar substances. This is due to its hydroxyl-ethyl combination of two functional groups that can efficiently hydrogen bond with other polar substances, while the ethyl component dissolves nonpolar substances. In a study done by Vongsak et al., they also used 70% ethanol as a solvent for extracting the leaves of M. oleifera and found high contents of crypto-chlorogenic acid and isoquercetin [4]. They also recommended maceration with 70% ethanol for the preparation of high-quality antioxidant extract from M. oleifera leaves for nutraceutical/pharmaceutical development. In another study, Rodríguez-Pérez and his team reported 59 compounds tentatively identified, which included phenolic acid derivatives and flavonoids as the most abundant [5]. However, they used a high-tech method that required an advanced laboratory equipped with ultrasound-assisted extraction (UAE). In most schools, such equipment is hard to come by, but teachers still develop techniques to teach students. Therefore, the aim of the present work was to determine the best solvent for the extraction of bioactive compounds from dried and ground moringa leaves with the intent to develop methods to teach students in a resource-limited laboratory environment.
The chemicals used in this experiment were acetone, chloroform, dichloromethane, diethyl ether, ethanol, methanol, and purified water. Food-grade moringa leaves were commercially purchased as “Hoja de Moringa” from a local grocery store, and the rest of the chemicals used were of analytical grade. Dried moringa leaves were ground in a clean electric mincer to a fine powder and soaked for 24 hours in an appropriate amount of solvent. The bioactive compounds were recovered at room temperature, and the fiber was separated from the mixture by 10 minutes of centrifuging.
Selection of the Solvent
The process for selecting the best solvent can easily get complicated. Using organic solvents like methanol and dichloromethane has been a major concern in the herbal industry. Because these toxic solvents are cheap and easily available on the market when used for extraction, they are documented to cause negative health effects. The U.S. Food and Drug Administration has posted federal regulations for the purity of solvents in herbal extraction for human consumption. Green Chemistry has labeled ethanol and water as excellent solvents and documented that they do not cause environmental or human health-related problems. In this study, a binary solvent of different combinations of ethanol-water with solvent polarity capable of extracting a wide range of organic and inorganic compounds was used. Other highly used extraction solvents like methanol, acetone, dichloromethane, diethyl ether, and chloroform were tested in the lab, but the binary solvent of ethanol-water was selected. This is because ethanol and water are more polar than acetone and have a higher dielectric constant. In water and ethanol, there is a stronger hydrogen bond, but ethanol can dissolve organic and inorganic substances. Both ethanol and water have hydroxyl groups, but ethanol is volatile, flammable, and has a medical application as an antiseptic and disinfectant. The table shows the data collected from the experiment (Table 1).
The mass of recovered solid compounds from dried moringa leaves is shown in (Figure 1). The solution was heated, and the recovered solid was weighed. When 20 mL of pure water was used as a solvent 0.213 grams of the compounds were recovered, and while 20 mL of pure ethanol was given, 0.007 grams of solid compounds were recovered. The slope of the curve decreases as the % of ethanol increases (Figure 2). shows the difference in the density of binary solvent with the moringa in the second solution. The graph peaks at 60-65% ethanol mixture (Figure 3). shows the recovered moringa extract. The approximate result from the extraction of the commercially dried moringa leaves gave the highest fiber and polysaccharide product (75.49%). The phytochemicals detected in the 4:1 methanol/water solvent were fats and waxes (3.085%), phenolics and terpenoids (12.975%), and alkaloids (8.450%). All these results indicate that the dried leaves have a large quantity of fiber and many vitamins and nutrients embedded in the plant. The presence of polyphenols and alkaloids explains the plant’s biochemical activities and medicinal action for therapeutic purposes.
The major components of moringa are vitamins (A, B, C, D, E, and K), polyphenols (flavonoids and phenolic acids), flavonoids, tannins, and saponins. In dried leaves, Gallic acid is the most abundant, with a concentration of 1.034 mg/g of dry weight [6]. Among the vitamins found in the dried leaves, vitamins A, D, E, and K are fat-soluble, while vitamin C and the B complex are water-soluble. Most of the polyphenols in the moringa-dried leaf are soluble in organic solvents, except phenolic acids. Many alkaloids are soluble in organic solvents and poorly soluble in water. Tannins are compounds that are soluble in water in large quantities because they are needed to protect plants from dangerous insects. The bioactive substances that have medicinal properties are glycosides, flavonoids, saponins, and tannins that are found in the dried leaf. The compounds that are soluble in water are vitamins B and C, phenolic acids, and tannins. Among the bioactive medicinal substances, glycosides are only soluble in water; saponins are soluble in organic substances and water, and flavonoids and terpenes are only soluble in organic solvents. For this and other reasons, the selection of the best solvent for moringa is complicated. Considering the pKa of ethanol at 16, and the pKa of water at 15.74, small quantities of ethanol can go through the following reactions. Water is more acidic than ethanol, and a very small molecule of ethanol reacts with water. This Bronsted-Lowery reaction will enhance ethanol’s ability to be a better solvent for organic compounds, while the production of water will enhance solvent capabilities.
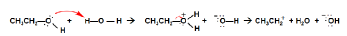
The maximum amount of extract used by the binary ethanol/ water solvent is in pure water, and the minimum is when pure ethanol is used as the solvent. Generally, the combination of ethanol and water is preferred over acetone, chloroform, ethyl acetate, methanol, n-hexane, and 2-propanol. Results indicate the binary solvent 60-65% ethanol-water combination is a better solvent for the herb. The moringa extract contains many functional groups, and the strong hydrogen bond of the binary solvent dissolved the components successfully. Even though moringa has serious side effects, the benefits outweigh the damage caused by the plant. Moringa is reported as a superfood and a strong medicine for a variety of ailments. Experience has shown that regardless of the side effects of moringa, it is good to consider herbal medicine that is complementary to food and other medicines and may not stand alone as a magical medicine. Caution must be taken as to the amount and frequency of this herb, and consumers may have to get physicians’ advice before taking moringa and be observed by a physician while taking moringa. The binary ethanol-water solvent has not added any toxicity but has enhanced the extraction process.
The author likes to acknowledge Lauren Stephenson and Thanya PerezRoldan for their help.


