Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Fatma B’CHIR1* , Ines Ben Amor2 and Olfa Mhasni3
Received: November 15, 2022; Published: December 06, 2022
*Corresponding author: Fatma B’CHIR, Natural Substances Laboratory (NSL), Institut National de Recherche et d’Analyse Physicochimique (INRAP), Pôle Technologique Sidi Thabet, 2020 Sidi Thabet, Tunisia
DOI: 10.26717/BJSTR.2022.47.007504
The essential oil isolated from northern Tunisian Citrus limetta peels, via hydro distillation, is analyzed by Gas Chromatography-mass spectrometry (GC-MS). Fourteen compounds of total oil volatile fraction are identified. The predominant components in the essential oil are limonene (49.2%), β-pinene (14.32%), ß-linalool (10.95%) and linalyl anthranylate (9.59%). Quantitative and qualitative variations in our essential oil molecular compounds and yields were observed compared to those from other origins. A study of the antibacterial activity of Citrus limetta essential is tested against five bacterial stains: Escherichia coli ATCC 8739 G (-), Salmonella typhimurium ATCC 14028 G (-), ATCC6538 G (+), Enterococcus feacium ATCC 19434 G (+) and one Candida albicans yeast ATCC 1023. The minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) values obtained showed that Citrus limetta essential oil has a great antimicrobial effect in all bacteria stains tested and particularly against yeast with value of 0.031 for MIC and 0.25 mg/ml for MBC. In addition, the antioxidant activity essential oil extract of Citrus limetta peel was evaluated using free radical scavenging test with DPPH.
Keywords: Tunisian Citrus limetta; Essential Oil; GC-MS; Antibacterial Activity; MBC and MIC; DPPH Scavenging Test
Citrus limetta (sweet lemon) is a plant belonging to Rutacaea family, growing widely in the south coast of Italia and Mediterranean regions as Tunisa (Rapisarda, et al. [1-2]). In Tunisia, Citrus limetta is called as a lime, limette or sweet lemon. C. limetta fruits are gaining popularity all over the world according to their sweet taste, their high vitamin C and antioxidant contents. Moreover, this variety of citrus is well known by the particularity quality and odour of its essential oils that constitute the basis of best perfumes. Because of its intense fragrance and organoleptic proprieties, the sweet lemon essential oil (EO) is widely used in cosmetic, pharmaceutical and confectionery industries (Mitropoulou G, et al. [3,2]). Sweet lemon dried peels and sweet lemon confit are with great importance in commonly Tunisian culinary art. These are majorly present in Tunisian traditional cakes and biscuits. The fragrance of sweet lemon zest is also used to give a pleasant taste to drinking water. Citrus limetta is well known by its multiple therapeutics’ activities in medicine, and in aromatherapy to improve mood and psychic disorders (Bagetta G, et al. [4]). In fact, sweet limon essential oil has been traditionally used in folk medicine for fever and parasitic diseases and disinfection of skin and aromatherapy, it is mostly appreciated for its antiseptic and antibacterial proprieties. This biological potential is attributed to its wealth in active compounds such as polyphenols, terpenes and flavonoides (Nogata Y, et al. [5]). However, the correlation between health benefits, biological activities and bio actives citrus chemical composition is still unclear (Djamel Djenane, et al. [6-7]). The aim of the present preliminary study was to determine, for the first time, the chemical composition of the EO extracted from fresh Tunisian sweet lemon (Citrus limetta) and to evaluate its biological potential. The antioxidant proprieties of this oil and its influence on Gram (-) and Gram (+) bacteria strains and also on yeast were tested.
Plant Material
Fresh Citrus limetta fruit were randomly collected, at maturity stage, from the northest region of Tunisia “Ras JBAL” and freshly peeled for analysis.
Essential Oil Preparation
About 200 g of fresh peels was subjected to hydrodistillation with 600 ml distilled water using a Clavenger-type apparatus. The oil obtained for 3 hours was separatedfrom the distillate, dried over anhydrous sodium sulfate and stored in sealedglass vial in refrigerator at 4C° until the moment of analysis in order to preventchanges in chemical composition. The extraction is done in triplicate
Essential Oil Extract Analysis
Gas Chromatography (GC): The essential oil composition was dertermined by Agilent 6890N Network GC system gas chromatograph fitted with a flame ionization detector (FID) and an electronic integrator,using a HP-5 (5% phenyl methyl siloxane) capillary columns (30 m _ 0.32 mm i.d.,film thickness 0.25 lm). The oven temperature was programmed from 50 _C to280 _C at 7_C/min; injector temperature: 220 _C; detector temperature: 240 _C;carrier gas: nitrogen (1.0 ml/min); sample of 0.2 μl was injected with split ratio of 1:100. The relativeamount of components in the oil was calculated by electronic integration of FID peak areas and normalized without the use of response factor correction. Retentionindices (RI) were determined relative to the retention times of a series of n-alcanes (C6–C22).
Gas Chromatography/Mass Spectrometry (GC/MS): The identification of the essential oil was carried out using Agilent 6890N Network GC system combined with Agilent 5975 B Inert MSD detector with electron impact ionization (70 eV). A HP-5-MS (5% phenyl methyl siloxane) column (30 m _ 0.25 mm i.d., film thickness 0.25 lm) was used. The column temperature was programmed to rise from 50 to 280 _C at a rate of 7 C/min. The carrier gas was helium adjusted to a linear velocity of 34 cm/s. Scan time and mass range were2.2 s and 50–;550 m/z, respectively. Samples (0.1 ll) were injected with a split ratio of 1:100. The components of oil were identified by comparison of recorded mass spectra with those of a computer library (Wiley 275 library and NIST98 database/ChemStation data system) and with those of authentic compounds. The identification was confirmed by comparison of their retention indices relative to (C6-C22) n-alkanes either with those of authentic compounds or with data published in the literature (Adams [8]). The identification of some components was also confirmed by co-injection of authentic standards under the same GC analysis, as well as by comparison of their retention indices with data from the Mass Spectral Library “Terpenoids and Related Constituents of Essential oils” (Dr. Detlev Hochmuth, Scientific consulting, Hamburg, Germany) using the Mass Finder 3 software.
Antimicrobial and Antifungal Activities
Microbial Species: Antimicrobial activities of Citrus limetta EO were investigated against four bacteria strains Escherichia coli ATCC 8739, G (-) Salmonella typhimurium ATCC 14028 G (-), Staphylococus aureus ATCC6538G (+), Enterococcus feacium ATCC 19434 G (+) and one Candida albicans yeast ATCC 1023).
Disc Diffusion Method: A suspension of the tested microorganisms was spread on the appropriate solid media plates and incubated overnight at 37°C. After 1 day, 4-5 loops of pure colonies were transferred to saline solution in a test tube for each bacterial strain and adjusted to the 0.5 McFarland turbidity standard (~108 cells/mL). Sterile cotton dipped into the bacterial suspension and the agar plates were streaked three times, each time turning the plate at a 60° angle and finally rubbing the swab through the edge of the plate. Sterile paper discs (Glass Microfibre filters, Whatman; 6 mm in diameter) were placed onto inoculated plates and impregnated with the diluted solutions in sterile water. Ampicillin (10μg/disc) was used as positive control for all strains except Candida albicans for which Nystatin (100μg/disc) was used. Inoculated plates with discs were placed in a 37 °C incubator. After 24 hours of incubation, the results were recorded by measuring the zones of growth inhibition surrounding the disc. Clear inhibition zones around the discs indicated the presence of antimicrobial activity. The test was run in duplicate.
Determination of the Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC): The MIC and MBC of R. Citrus limetta oil extract were determined using a broth dilution method as previously described by (Cosentino S, et al. [9]). Overnight broth cultures were diluted in peptone water (0.1% (v/v)) to obtain working culture (105 CFU/ml). Serial dilutions, ranging from 0.009 to 2 mg/ml of the extract, were used, including one growth control (TCS) and one sterility control (appropriate medium þ tested extract). Tubes were incubated for 24 h at 30 or37 _C and the MICs and MBCs were determined. Microbial growth was indicated by the presence of turbidity and a ‘pellet’ on the tube bottom. MICs were determined presumptively as the first tube, in ascending order, which did not produce a tube bottom. To confirm MICs and to establish MBCs, 10 ml of broth was removed from each well and inoculated on TCS plates. After incubation, the number of surviving organisms was determined. The MIC was the lowest concentration which resulted in a significant decrease in inoculums viability (>90%), while the MBC was the concentration where 99.9% or more of the initial inoculum was killed.
Antioxidant Activity
Free Radical –Scavenging Activity: The diphenyl picrylhydrazyl radical (DPPH) scavenging capacity was measured as previously described by (Brand-Williams, et al. [10]). 1 ml of essential oil extract (at different concentrations rainging from 20 to 80mg/ml) was mixed with 0.5 ml of DPPH methanol solution (0.1Mm). The mixture was shaken vigorously and standing at room temperature for 30 minh in the dark and then the absorbance was measured at 515nm. The scavenging activity was estimated using the following equation: scavenging effect (%) = 100*(Ac-As/Ac), where Ac is the absorbance of the control reaction (contains all reagents except the test sample). And as it is the absorbance of the tested sample. The antiradical activity was finally expressed as 50% inhibiting concentration (IC50). A lower IC50 value corresponds to higher antioxidant activity of essential oil.
All samples were analyzed in triplicate.
Chemical Composition of Citrus Limetta Essential Oil
Our result showed that the average of three measurements of the extraction yield of EO from zests of fresh Citrus limetta fruits grown in Tunisia (Ras Jebel, Northeast coast), harvest in March at maturity stage and measured after a 3-hour hydro distillation is 0.86±0.11 % calculated onfresh weight (Foschi R, et al. [11]). This result was higher than obtained in Tahran (Kamaliroostaa L, et al. [12]) and in Pakistan (Javed S, et al. [13]) studies which reported an average yield of 0.54% and 0.313%, respectively, for Citrus limetta EO. Mexico study reported also a lower yield of 0.26±0.02% from steam distillation of C limetta mature fruits (86). Conversely, a higher EOs yield of 1.0% was obtained from fruits of C. limetta Risso collected in Italy (87). The variation in citrus peels oil extraction yields may be attributed in part to the time and the method used for extractions as well as the maturity stage of fruits collected. (Table 1) showed that monoterpenes were the most important constituents with 80.0% of the total followed by 16.9% oxygenated monoterpenes and 3.0% sesquiterpenes. Among monoterpenes, half of them was limonene (49.2%) followed by ß-pinene (14.06%), camphene (3.62%), α-pinene (1.41%), and α-terpinene (0.53%). Oxygenated monoterpenes were represented by ß-linalool (10.95%), linalylanthranilate (9.59%), neryl acetate, (3.57%), nerol (0.72%), terpinen-4-ol (0.59%) and geranial (0.57%) while β--bisabolene (1.55%), α-bergamoten (0.89%) and ß-caryophyllene (0.38%) were identified as sesquiterpenes (Figure 1).
Our results are quite similar from those obtained by literature (Arafat Y, et al. [14]) who reported that the most important compound of Citrus limetta EO was limonene with quantitative variation. It is present in percentages ranging from 49% to 95%. Our EO Citrus limetta chemical analysis is also quite similar to chemical analysis of Citrus lemon variety EO, in molecular composition, reported by Djamel Djenane 2005. By studding the chemical composition of EO Citruslimetta, (Moufida S, et al. [15]) confirmed that this essential oil consists mainly of limonene. However, by analyzing other investigations, we observed that some chemical compounds found in our Citrus limetta EO were absent in sweet citrus EO reported by (Essadik FZ, et al. [16-17]) such as: gamma-3carene, allo-ocimene,), Linalylanthranilate, neryl acetate, geranylacetate,trans α- Bergamotene which is present in smaller percentage 0.89% as a minor compounds. In other hand, we have reported that some minor molecular constituents are present in Citrus limetta EO chemical profile of some studies but totally absent in our extract. We noted the gamma terpinene (Vinodhini M, et al. [18-19]), alpha humulene, famesol (Benhada. H, et al. [17]), octan-1-ol (JabriKarouiI, et al. 2013), α-Terpinyl acetate (Benhada H, et al. 2016), l imone oxide (Sanei-Dehkordi A, et al. [20]). Many studies noted the absence of those compounds in Citrus limetta EO mainly the Nerol and Geraniol (Gancel AL, et al. [21]). So, our EO sample present a specific chemical profile characterized by the presence of some particular constituents which are absent in other sweet lemon EO studies. According to (Senatore F, et al. [22]) this qualitative and quatitative variations profile of sweet lemon EO may be due one or combinaison of those factors: genetic, age and environmental climate of the plant. Add to these factors, it is also noted that maturity of the fruit has a significant impact on the chemical composition of the oil obtained (Minh Tu et al. [23]).
Screening of Essential Oil Citrus Limetta
Antimicrobial Activity
The screening of the antimicrobial activity in vitro of EO Citrus limetta was studied using the agar diffusion disc. The results were recorded by measuring the zones of growth inhibition surrounding the disc. Clear inhibition zones around the discs indicated the presence of antimicrobial activity (Figure 2). (Table 2) The sample extract exhibited a good effect against all pathogen’s strains tested. However, this effect is heavier on bacteria gram (+), it concerns Staphylococcus aureus ATCC 6538 and Enterococcus feacium ATCC 19434with inhibition zone diameters 22 and 32 mm, respectively (Table 2). Therefore, the two-gram (-) bacteria references were less sensitive against the tested extract. Our extract has also an important effect on Candia yeast reported by an inhibition diameter of 28mm (Figure 2) In order to approve the antimicrobial activities potential of Citrus limetta EO extract, a micro dilution assay was done.Micro Dilution Assay: The results of MIC and MBC are shown in (Table 2). Among the five pathogens strains tested, a better antimicrobial performance was obtained against Candida albicans on a of 0.031mg/ml for a MIC and 0.25mg/ml for a MBC. An inhibitory effect of Citrus limetta was observed until a MIC dilution of 0.25 and 0.125 for Enterococcus feacium and Staphylococcus aureus, respectively. The MIC and MBC values obtained showed that Citrus limetta EO has a great antimicrobial effect, particularly against yeast and Gram-positive bacteria. The present study is not consistent with those reported by (Smith Palmer, et al. [24]) who they demonstrated that EO lime was more effective to gram(+) than gram(-) bacteria, our results showed that bacteria gram(-) were more sensitive to Citrus limetta extract oil than the gram(+). The work of De Biller beck, (2007), carried out on the antibacterial activity of the EO of lemon, showed diameters of inhibition of the growth of the two strains E.coli and Staphylococcus aureus of the order, respectively of 8 and 12 mm. These measurements are far below our results. According to (Oussalah, et al. 2007) the antimicrobial power of EO is directly related to several parameters, namely: the nature of the majority compounds of EO, the choice and the physiological conditions of the microorganisms studied. This antimicrobial activity can be determined by the effect of a single compound or by the synergistic or antagonistic effect of various compounds, mainly monoterpene compounds (Deba, et al. 2008) (Figure 3).
Table 2: Inhibition Zone Diameter (IZD), Minimal Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC) of Citrus Limetta Essential Oil Against Five Reference Strains Strains.
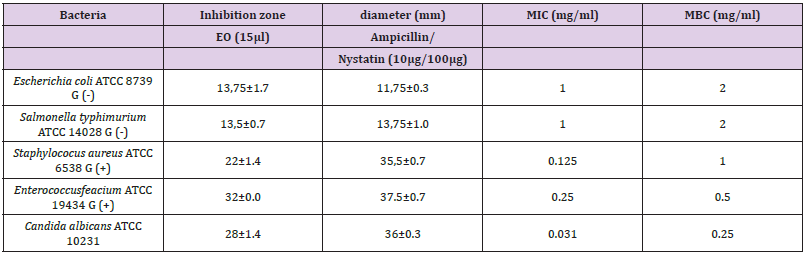
Figure 2 Zones of growth inhibition (mm) of Citrus limetta essential oil (15μl) against four bacteria and one yeast. 1. (+): Ampicillin positif control for bacteria 2. (+): Nystatin positif control for yeast.
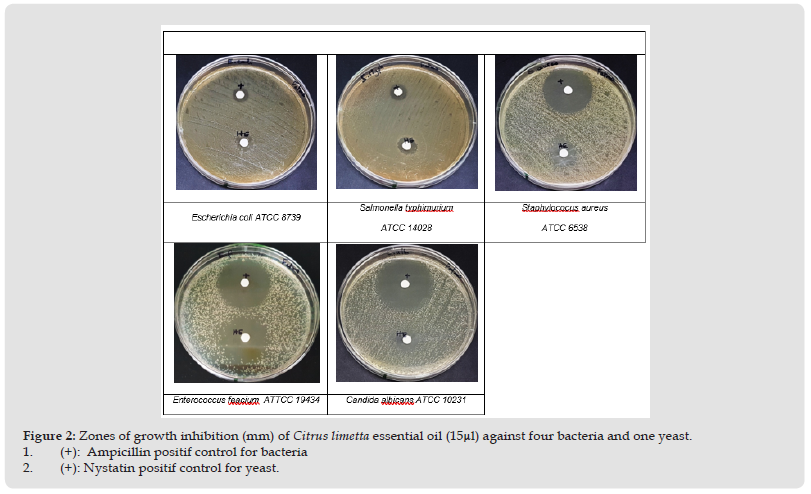
Figure 3 Detrmination of Minimal Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC) of Citrus Limetta Essential with Elisa Microdilution Method.
The strong antimicrobial activity of t our citrus peels EOs, revealed in our study, would be attributed to their richness of monoterpenes compounds, according to GC-MS analysis, in particular the ß-pinene and the oxygenated monoterpenes. In fact, until know the significant correlation between chemical composition and antibacterial activity was not well established.Some studies (Djamel Djenane, et al. [6,25]), (Zhang Z, et al. [26-31]) explained that citrus, with major constituent limonene, were less effective as an antimicrobial agent. This deduction is not coherent with the present study, knowing that the major constituent of our citrus extract was limonene and present a great antimicrobial potential as an EO. So, the compounds present in larger proportions may not necessarily be responsible for the antibacterial activity of EO but the participation of less abundant constituents must be considered. Therefore, it is more interesting to study the antibacterial propriety of each compound separately, the antimicrobial proprieties are in partly related to their lipophilicity synergy with the permeability of cellular membranes. It should be noted that there was a considerable variation between antimicrobial and chemical composition of several EO extract, this variation was observed, even within the same species.
This variation can be attributed to many factors as: sol composition, cycle of vegetation, age of maturation as well as the chemical composition of citrus oil or plant extract in fact, the variation on chemical constituents depend on the environmental conditions of plant and the variation on antimicrobial potential may be explained by the method used to assess the antimicrobial activity (method of disc diffusion, micro dilution,) the result obtained may be different. All these factors should be taken in consideration to explain conflicting results observed from different studies.
Antioxidant Activity: DPPH Test
DPPH was a free radical that accepts an electron or hydrogen radical to become stable molecule. DPPH radical scavenging was one of the commonly used methods to evaluated antioxidant activity of plant extract. (Figure 4) illustrated the DPPH scavenging effect percentage EO at different concentrations of Citrus limetta EO. The 50% inhibition concentration (IC50) was determined from the curve of (Figure 4). The IC 50indicates the antiradical activity of extract. The IC50 value obtained for our oil extract was 33±0.06 mg/ml. this result showed a moderate antioxidant activity of Citrus limetta EO. Several others anitioxidant tests should eventually effectuated to estimate the antioxidant potential of this citrus variety EO.
The major volatile constituent of our zests citrus oils was limonene. This finding was in accordance with many research focused on citrus oils composition whom declared that citrus genus EO is consisted mainly of limonene with an overage ranging from 71% to 91% (Minh Tu, et al. [23]). In conclusion, our results showed that Tunisian Citrus Limetta essential oil was rich in limonene and the GC-MS analysis demonstrate da distinguished chemical profile with variation in abundance of volatile fraction compounds. The variation concerns also the quality of oil explained by the presence of some compounds in one oil and absence of them in other. Via the micro dilution assay, Citrus limetta EO present a potent antimicrobial and antifungal activity. This preliminary result suggested that the Tunisian Citrus limetta EO possesses antimicrobial and antifungal properties, beside to distinguished chemical profile, can be used therefore as a potential source of active ingredients for food and pharmaceuticals industries.


