Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Loushambam Samananda Singh1* and Laimayum Amarnath Sharma2
Received: November 23, 2022; Published: December 01, 2022
*Corresponding author: RLoushambam Samananda Singh, Institute of Pharmacy, Assam Don Bosco University, Tapesia, Sonapur, Assam, India
DOI: 10.26717/BJSTR.2022.47.007495
Background and Aims: The purpose of this study was to see if there were any differences in leptin levels and lipid profiles between non-obese and obese individuals, as well as to see if there was any relationship between leptin levels and lipid profiles in obese subjects in the Manipuri population.
Methods: This is the comparative cross-sectional study design. The research was undertaken in the Department of Physiology, Regional Institute of Medical Sciences, Imphal between July 2018 and July 2021. There were 277 non-obese and 323 obese participants in the study. The ELISA method was used to determine serum leptin levels, and the CHOD-PAP and GPO-PAP procedures were used to determine the lipid profile. Body fat percentage (body fat %) was also measured to check if there were any differences between the groups. A student t-test (unpaired) was utilized to compare the findings of different groups. In addition, for correlation analysis, the Pearson correlation coefficient (r) was used. Significant was defined as a P<0.05.
Results: There were significant changes in leptin levels between non-obese and obese individuals (P<0.05), but no significant changes in lipid profile. Body fat percentage (body fat %) and leptin both have significant positive correlations (P<0.05) with body mass index (BMI). But lipid profile had no associations with leptin and BMI.
Conclusion: In this study, leptin was associated with obesity, while lipid profiles revealed no relationship between leptin and obesity.
Keywords: Leptin; Lipid Profile; Obese; Manipur
Abbreviations: BMI: Body Mass Index; HSL: Hormone-Sensitive Lipase; LDL: Low-Density Lipoprotein; VLDL: Very Low-Density Lipoprotein; GPO: Glycerol Phosphate Oxidase; SD: Standard Deviations
Leptin, a highly conserved 16kD hormone was discovered in the early 1990s as one of the adipocytokines produced by the white adipose tissue and also the discovery of its receptors in the central neuronal and extra neuronal peripheral tissues followed quickly [1]. Its main function is to maintain a minimum level of energy storage under caloric restriction and to delay the accumulation of body fat by reducing food consumption when there is an excess of energy intake [2]. The mechanism of leptin action is by sensing the energy balance i.e., increases in synthesis with the expansion of fat cells (which directly relate to the state of nutrition). This leads to the inhibition of hypothalamic Neuropeptide Y synthesis and release from the arcuate nucleus of the hypothalamus whenever excess energy is stored or body fat mass occurs [3]. An increase in serum leptin level is associated with an increase in body fat mass [4]. But the regulatory effect of leptin is restricted to a certain serum level only as there is a threshold plasma leptin concentration (about 25ng/ml) above which saturation of leptin occurs across the bloodbrain barrier in the Cerebrospinal Fluid despite high serum leptin level. It is yet to investigate whether the threshold level differs in ethnic and racial populations. It is also of great interest that in rodents and humans, loss of leptin signaling following gene mutations of either leptin or its receptors results in increased food consumption, and a decrease in energy expenditure leads to obesity.
This event could also lead to other neuroendocrine disorders like infertility, hypothyroidism, decreased growth rate, etc because of its involvement in many neuroendocrinal functional pathways [5,6]. The excess fat mass in such obese subjects is because of the deposition of hyperplastic and hypertrophic adipocytes secreting high levels of leptin. Defective leptin receptors can be a reason in other cases of obesity because of the development of ‘leptin resistance’ in which signaling of leptin is attenuated [7]. A very large proportion of ingested fats are stored as triacylglycerols, a water-insoluble long-chain hydrocarbon large molecule, in the fat droplets of the adipocytes as energy stores. Triglycerides (TG) are the ester derivative form circulating in the blood and released as energy substrates for the tissues and organs of the body in the postabsorptive state. Its serum level positively relates to a high-fat diet and low utilization. Apart from triglycerides various biologic lipids with different functions have been identified. Cholesterol (TC) is another lipid, the biosynthesis of which increases in high energy intake and circulates in plasma with high serum level. Lipoproteins are complexes of proteins and lipids held together by noncovalent bonds and are classified by the site of their assembly, apoprotein dominance, and types of lipids [8]. Lipoproteins are complexes of proteins and lipids held together by noncovalent bonds and are classified by the site of their assembly, apoprotein dominance, and types of lipids [8].
Major transporter of lipids i.e., TG, cholesterol esters, TC, and phospholipids. Approximately 80% of the fatty acids liberated from triacylglycerols by lipoprotein lipase are normally taken up by muscle and adipose tissue, the remaining is cleared by the liver. Control of the hydrolysis of TG stored within the adipocyte is carried out by the enzyme hormone-sensitive lipase (HSL). Increased insulin rises in the post-ingestion period and also with increased leptin secretion because of increased adipocyte size is accompanied by the inactivation of HSL leading to reduced lipolysis. When dietary energy intake occurs and secondary to insulin action is accompanied by a shift in adipose tissue metabolism in which LPL at the endothelial surface is activated. Alteration of the LPL might be a cause of excess fat deposition in obesity. Analysis of serum level of lipoproteins, namely Low-density lipoprotein (LDL), Very Low-density lipoprotein (VLDL), and High-Density lipoprotein (HDL) give the highly valuable status of serum lipids regulation in the body and operative mechanisms behind obesity. The abnormal condition is termed dyslipidemia. Most of these dyslipidemias are hyperlipidemia where most of the lipid levels shift towards the higher limits of a statistically determined normal reference range and beyond [9]. Lipodystrophy is another condition where adiposity is very much reduced, but the lipid accumulates excessively in non-adipose cells and tissues like skeletal muscles and the liver.
Leptin treatment reduces lipid deposition in non-adipose tissue and improves the severity of diabetes in lipodystrophy patients and in rodents [10]. Lipodystrophic rodents and humans have low to no adipose tissue, which is hyperleptinemia, which also shows hepatic steatosis, and hyperlipidemia, which shows better results by leptin [11,12]. Thus, leptin has linked to lipid metabolism which is independent of body weight effectively. The liver plays a major role in lipid metabolism in which leptin acts to exert its metabolic effects in the liver due to receptors of leptin being available in the liver [13,14]. Leptin induces changes in gene expression in ob/ob mice along with lipid metabolism involved in the liver [15]. Hepatic steatosis can be reversed in ob/ob mice when leptin is treated, ultimately leptin may directly influence the liver [16]. The discovery of leptin receptor gene expression and its isoforms in peripheral tissues led to speculation in lipid metabolism by the role of leptin, without effects in the hypothalamus [17-20]. Leptin induces lipolysis when treated in animals, and subsequently inhibits lipogenesis and the observed reduction of adipose tissue explicitly by stimulating fatty acid oxidation. In addition, acute administration of leptin in rats developed hypertriglyceridemia, and triglyceride uptake decreased significantly in muscle and adipose tissue [21], although this change was not related to variations in lipogenesis. However, how leptin directly affects lipid metabolism is not well understood.
Manipur is a northeastern Indian state with a population who are mongoloids with distinct ethnicity, cultural, social, and dietary traits who dwell at higher altitudes. It’s possible that the discovery of a specific pattern of energy metabolism, physical build, and fitness piqued people’s attention. Several investigations in different ethnicities with varying genetic backgrounds have found that different grades of BMI have varied circulating leptin and lipid profiles. However, there are few studies on overweight/obesity in this part of India’s northeast. The purpose of this study is to acquire more about circulating leptin and its association with lipid profiles in Manipuri individuals of various BMI levels.
This was a cross-sectional study conducted in the Department of Physiology at the Regional Institute of Medical Sciences in Imphal, Manipur, India, between July 2018 and July 2021. The Institutional Ethical Board granted permission to perform this study (Ethical Approval Code: A/206/REB/Prop (SP)70/46/2019). A total of 600 participants were recruited from the Manipuri population, both sexes, who are Indo-Tibetan descendants of the mongoloid race. The recruits gave their informed consent to participate. Personal information, relevant history, and vital data (Proforma) were obtained to determine the subjects’ eligibility for participation in the study. Each patient was allocated a certain day as an appointment day for reporting in the early morning when blood samples would be drawn. In addition, instructions and procedures to be followed during the pre-test hours were provided.
Inclusion Criteria
All participants, both sexes, between the ages of 20 and 65, with physical structural traits that appear to be well-built or overweight.
Exclusion Criteria
Diabetes mellitus, overt hypothyroidism, genetic metabolism defect, cardiovascular illness, renal, psycho-neurotic, cancer, and other metabolic conditions that are likely to impact nutritional health were excluded. Pregnant women and lactating mothers are also barred from taking part.
Group Classification
The whole study population was classified as non-obese (BMI - < 25 kg/m2) and obese (BMI - ≥ 25 kg/m2) based on different grades of BMI as determined by WHO criteria for Asia-Pacific recommendations [22].
Blood Sample Collections
Blood samples were drawn in the early morning, between 7 and 9 a.m., before any food ingestion (as had been informed beforehand). Under aseptic conditions, 8-10 ml of blood was taken from the antecubital vein in a plain vial, and serum was separated after centrifugation at 3000 rpm for 10 minutes.
Sample Size
According to National Family Health Survey, 2015-16 (NFHS-4) [23], 26% of women and 20 % of men are overweight or obese in Manipur. The sample size is computed using the prevalence of 26% and the 95% confidence interval [24].
Anthropometric Measurement
Height was measured barefooted using a portable Stadiometer following the definition of erect posture with an error restricted nearest to below 5 mm. Weight was taken barefoot and in a short, light dress on a Tanita weighing scale, with an error nearest 500 gm [25]. BMI was calculated as the ratio of body weight (in Kilogram) to the square of the body height in meters. The body fat percentage (BF %) was calculated indirectly by using the Deurenberg equation [26].
Body fat percentage=1.2(BMI ) + 0.23(age) −10.8(sex) − 5.4
With age being in years and sex being designated as 1 for males and 0 for females.
Biochemical Measurements
Serum Leptin: Biochem Diagnostics Canada Leptin Reagents were utilized to estimate blood leptin concentrations using an enzyme-linked immunoassay approach with an ELISA Microplate Reader, Thermoscientific’s Multiskan FC.
Serum Lipid Profile:
Serum TC: Erba Manheim Cholesterol Reagents were used for the determination of serum cholesterol concentration by CHODPAP method using Clinical Biochemistry Analyser Erba EM 200.
Serum TG: Erba Manheim Triglycerides Reagents were used for the estimation of serum triglycerides concentration by enzyme glycerol phosphate oxidase (GPO) method using Clinical Biochemistry Analyser Erba EM 200.
Serum HDL: The Clinical Biochemistry Analyzer Erba EM 200 was used to determine the serum HDL concentration using Erba Manheim direct HDL reagent.
Serum LDL and VLDL: Serum LDL and VLDL were calculated by using the Friedewald calculation method as follows: -
LDL(mg / dl) = TotalCholesterol − HDL −C − (Triglycerides / 5)
where “triglycerides/5” is used to represent very low-density lipoprotein
Statistical Analysis
Statistical analysis was performed using SPSS software version 26. All data were presented as mean ± standard deviations (S.D.). Student t-test (unpaired) was used to compare the results of different groups. In addition, the Pearson correlation coefficient (r) was used for correlation analysis. P value <0.05 was considered significant.
The study enrolled a total of 600 individuals, with 218 men and 382 women. The demographic profiles of the different sexes in the current study are shown in (Table 1). The study included 277 non-obese subjects and 323 obese subjects. (Table 2) shows the biochemical parameters of non-obese and obese people. (Table 3) shows a comparison of the male and female individuals’ demographic profiles and biochemical data in relation to non-obesity and obesity grades. Females had higher BMI, body fat percentage, and leptin levels than males, although lipid profiles did not differ significantly between the sexes. No statistically significant correlations were found between the BMI and lipid profile. Leptin and body fat % has been demonstrated to be significantly correlated, but not between leptin and lipid profile. BMI was found to have strong positive associations with body fat percentage (Figure 1) and leptin levels (Figure 2).
Note: *Significant at P<0.05, **significant at P<0.01
Note: *Significant at P<0.05, **significant at P<0.01
Table 3: Comparison of the Demographic profile and biochemical parameters of the male and female subjects about the non-obesity and obesity grading.
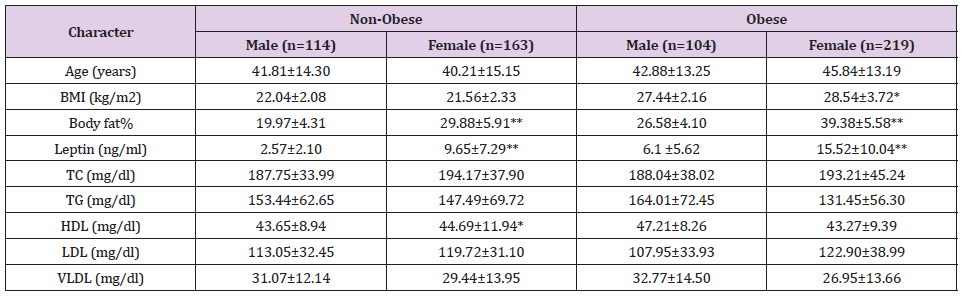
Note: *Significant at P<0.05, **significant at P<0.01
The distinctive characteristic of the state and the weightage of conducting this study is that although the indigenous people are not widely exposed to the westernized dietary and cultural lifestyle, economically in poorer strata at the national level and still subsisting mainly on green fresh vegetables, fish, fruits, cereals with their mainly agrarian lifestyle, in disbelief the state has been placed in the 15th rank in the national list of states with high obesity prevalence (NFHS-2015) [23]. In this study during the investigation, whenever any incidental disease mainly related to metabolism like diabetes mellitus, glycogen storage disease, bulimia, etc. is detected, they are excluded abruptly. Body fat % was found significantly higher among obese in relation to non-obese (P<0.00). Mean leptin level was increased significantly in obese than in non-obese in this study (P<0.00) and is correlated with its larger adiposity (r=0.576, P<0.00). The result of this study is consistent with the study by Concidine, et al. [27], according to whom obese subjects had a leptin level four times higher than non-obese subjects, attributing body weight maintenance could be one of the major positive approaches to control hyperleptinemia. One of the findings reported by Dasgupta, et al. [28], studied in the south Indian population showed a significant difference in leptin levels (11.14 ± 7.82 ng/ml of nonobese and 30.71 ± 15.53 ng/ml of obese). In our study, leptin levels were 6.74±6.72 ng/ml and 12.55±9.89 ng/ml in non-obese and obese groups, respectively.
The range of leptin levels among non-obese and obese was double- fold higher than our findings as well as elevation of leptin in obese was greater in their study could reckon differences in genetic, racial, food cultural background which are likely contributory factors that lead to variations in adiposity and leptin level among the different population. Body weight is regulated by a set point mechanism [29], and by the production of leptin from adipocytes with its sensitization at the hypothalamus determining the set point equilibrium [29,30]. Thus, the determination of genetic factors in the leptin gene may be involved in the regulation of leptin production and could be an important set point to define in obese individuals. Several studies in humans [31,32], and rodents [33-35], claimed that serum leptin concentrations are regulated by direct changes in the expression of the ob gene. It seems consequent that changes in body fat are translated into changes in serum leptin at the level of ob gene expression. The present study can be in line with this hypothesis which has been also tested by Ravussin, et al. [36], who claimed that the Pima Indians, an ethnically distinct race has relatively low plasma leptin concentration preceding weight gain. Moreover, leptin resistance develops during the early stages of obesity and strongly influences the metabolism of muscle fatty acids and insulin sensitivity [37].
Furthermore, leptin concentration is not dependent on adipocyte size, most probably due to factors unrelated to adipose tissue mass as several cytokines regulate leptin expression [38]. Under circumstances of reduced caloric intake, accompanying lower fasting serum insulin concentration and induced activation of glucocorticoids that mobilize energy stores may alter serum leptin secretion, hence resulting in variability in its serum level. Thus, all these phenomena reckon the importance of analyzing the serum leptin level in both non-obese and obese considering the unique food cultures, food habits, and likely genetic makeup that prevails in different ethnicity. Differences in serum leptin levels between male and female groups and also between obese and non-obese subjects among each sex group in this study revealed the commonly observed trend of the higher level of serum leptin in the female with different BMI when compared to their male counterpart. The increased leptin level in female in comparison to males are considered a global observation that does not relate to ethnicity, race, and food cultures. However, it can be observed that the magnitude of the rise in quantitative values of serum leptin levels in obese females and males in comparison to non-obese in this study appears remarkably less when compared to similar studies done elsewhere [39-41].
All the studies claimed the levels of leptin levels were found significantly higher in women after the correction was made for BMI or fat mass. On the other hand, this narrower difference in serum level between obese and non-obese reminds the probability of inclusions of cases with other underlying mechanisms like defective leptin signaling cascade or central hypothalamic lesions in which impaired leptin production is not associated with human obesity. A very important component aspect i.e., the associated risk of the degree of adiposity to mortality following various disease complications makes this segregation complex and overlapping. This is because the degree of excess adiposity can differ among individuals who may have a difference in susceptibility to these complications. Considering many debatable overlapping degrees of adiposity fit to be labeled as obese and non-obese, many standards of references are suggested and used by various researchers in their obesity-related studies [42]. This reflects a need to study if uniqueness in adiposity character exists in different types of BMI of a known classified system. The presumptive attributable factors of ethnicity, race, culture, and unique lifestyles can lead to the degree of fatness and associated changes in the neuroendocrine factors adapting to different dietary nutrients which are the basis behind the regulation of energy balance and body weight.
The rise of leptin levels with higher TG levels has been observed in numerous published studies in obese people, and the relationship of high TG levels with overweight and obesity has also been proposed [43,44]. Suspecting high carbohydrates and fatty diet leading to an increase in TG and TC which causes leptin resistance may contribute to the reason behind obesity. Herbst, [45] did a study as her thesis work and concluded that reduced TG levels by restricting high carbohydrate and fat both or independently promises a means to control obesity. However, the levels of TG, TC, HDL, LDL, and VLDL were shown to be insignificantly different between the non-obese and obese groups in the current study. This study agrees with that of Tagliaferri, et al. [46], who studied leptin levels and lipid profiles in euthyroid obese and subclinical hypothyroid obese subjects and found no significant abnormality in lipid profile. This study also found serum leptin levels not correlating with serum lipids including TG level and many similar observations were published by Idris, et al. [47], and Oguz, et al. [48]. According to a previous study, leptin, and BMI are associated to lipid profile independently [49,50]. Our research, on the other hand, observed no relationship between lipid profiles and obesity, which is consistent with earlier findings [51].
The conflicting findings of lipid profile especially of the Triglycerides concerning leptin levels in obesity encompassing various energy metabolism conditions reckon the need to consider the difference in physical built due to racial, genetic, cultural, dietary components and activity trends related to lifestyle. This study suspects all or any of which can be a probable closely related determinant to the operational character of the specific sensor (unique set point for leptin threshold to cross the blood-brain barrier) and its energy regulation. The Manipuri people are known to consume carbohydrates in the form of rice as the main staple food, with minimal levels of direct saturated fat in their diet. The accumulation of dietary fat in adipose tissue causes the weight increase that marks the progression of obesity. At the same time, de novo lipogenesis from carbohydrates would be very unfavorable to increasing body fat stores [52]. Does the finding suggest that other pathways occur for the cause of obesity or is the sensitivity of TG to cross the bloodbrain barrier to effect suppression of leptin action in the hypothalamic blunted? Further, the finding of the present study is consistent with Hussain, et al. [53], when considering gender differences. Levels of differences in lipid profile between males and females have been reported with the suggestion of sex steroids as the influencing determinants, but the mechanism remains inconclusive.
This study also found that females have a greater value than males, however, the difference is statistically insignificant. The research is restricted to a demographic with a small sample size that does not adequately represent the overall population. For detailed correlations among adiposity indices, it is necessary to add some parameters such as dietary and genetic factors in a larger sample size population and would like to propose comparisons between persons who successfully defend their weight and people who gain weight more easily to gain a better knowledge of obesity. The current study, on the other hand, looks at the interrelationships of obesity variables at different BMI levels among Manipuri people.
In this study, leptin was found to have a strong relationship with obesity. The lack of substantial correlations between leptin and lipid profile in obese individuals, contrary to most previous studies, could be attributable to nutritional or genetic variations among this indigenous population. Alternatively, there may be a threshold at which the levels shift and turn abnormal in obesity.
We would like to express our gratitude to MDRU, Regional Institute of Medical Sciences, Imphal for their support of this study.
None.


