Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Girum Tefera Belachew* and Paramesh Hanumanthaiah
Received: November 09, 2022; Published: November 23, 2022
*Corresponding author: Girum Tefera Belachew, Department of Biotechnology, College of Natural and Computational Sciences,Debre Birhan University, P.O.Box 445, Debre Birhan, Ethiopia
DOI: 10.26717/BJSTR.2022.47.007473
Background: Typhoid (enteric) fever is a significant medical condition. The
World Health Organization uncovered that around 21 million cases and more than six
hundred thousand yearly died from typhoid fever. Non-industrialized countries share
the most elevated weight because of rapid population growth, increased urbanization,
and inadequate safe water and healthcare systems.
Objective: This study was intended to show seroprevalence for Salmonella Typhi
infection among patients attending the student clinic at Debre Birhan University.
Methods: A retrospective cross-sectional study was conducted on Widal test results
registered from December 2019 to June 2021 at Debre Birhan University student
clinic. After official permission was obtained from the head of the student clinic,
the data were retrieved from medical records (registry books) of the laboratory unit
for the study period. The data were gathered and saved in excel sheets which were
further analyzed by statistical package for the social sciences (SPSS) software version
20. Results. Our study revealed the seroprevalence of typhoid fever was 69.23%,
(95% confidence interval [CI] = 195.58-605.42%). Among 1300 males (56.17%), 998
(76.77%) were reactive for O and H antigens (571 for O-antigen, and 427 for H-antigen,
M ± SD = 499 ± 101.82), 302 (23.23%) were non-reactive for O and H antigens
(231 for O-antigen and 71 for H- antigen, M ± SD = 151 ± 113.14). Of 1014 (43.82%)
females, 604 (59.57%) were reactive for O and H antigens (319 for O-antigen and 285
for H-antigen, M ± SD = 302 ± 24.04), 410 (40.43%) were non-reactive for O and H
antigens (135 for O-antigen and 275 for H-antigen, M ± SD = 205 ± 98.99).
Conclusions: Typhoid fever is a significant general health problem in Ethiopia.
Indeed, even today, an enormous number of cases are affirmed through a single semiquantitative
slide Widal test, despite its known low investigative value.
Keywords: Debre Birhan; H-Antigen; O-Antigen; Prevalence; Salmonella Typhi; Widal Test
Enteric fever is a significant medical condition. Reports by the World Health Organization uncovered that around 21 million cases and more than six hundred thousand yearly died from typhoid fever happen worldwide. Non-industrialized countries share the most elevated weight because of rapid population growth, expanded urbanization, and inadequate safe water and healthcare systems [1,2]. Salmonella Typhi separation from blood, bone marrow, urine, or stool is the most reliable method of affirming typhoid disease. However, this needs laboratory facilities and specialized preparation that are not plausible for most primary medical services in developing nations [3-5]. Thus, most typhoid diseases are analyzed on clinical grounds and treated possibly. However, as its clinical indications are comparative with numerous different bacteria, it might lead patients to get excessive and unseemly antimicrobial treatment [6].
In many non-industrialized nations, the Widal test, first presented by F Widal in 1896, is generally utilized to determine typhoid fever. Widal test is somewhat less expensive, simple to perform, and needs little preparation and low modern equipment [7,8]. This test relies upon agglutination reaction between Salmonella typhi somatic Lipopolysaccharides O antigen (TO) and flagellar H antigen (TH). These antigens are present in numerous other Enterobacteriaceae; thus, its test values have been argued for a long time [9,10]. Besides, understanding of results has additionally been a problem as various cut-offs points have been stated from different areas [11]. Furthermore, patient treatment can’t hang tight for results acquired with convalescent-phase samples. Thus, the treatment choice is made based on the outcomes achieved with a single acute- phase sample [12].
Due to the low predominance of typhoid fever, availability of safe drinking water, better research centers to identify the microbes which cause enteric fever, and the low sensitivity and specificity of the Widal test, the test is at this point not utilized as an analytic test in industrialized countries [13]. Nonetheless, it is the most routine test in emerging nations. Widal test prescribes a slide test to be utilized for screening only and positive outcomes to be affirmed by tube titration technique; though, widal titer results require 18-24 hours, the finding is done based on the slide agglutination outcomes, which are accessible within one minute. However, this might prompt bogus determination of typhoid fever, pointless antibacterial treatment, and the rise of medication-resistant strains [2,14]. In Ethiopia, the determination and treatment of typhoid fever is by Widal test (Slide agglutination). Therefore, the present study was designed to show the seroprevalence of enteric fever among patients attending the student clinic at Debre Birhan University, North Shewa, Ethiopia (Figure 1).
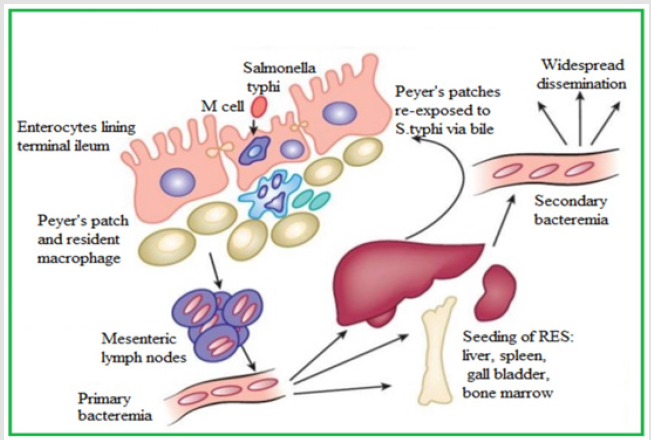
Figure 1.Pathogenesis of Enteric fever https://renumadan.com/2018/04/01/facts-one-must-know-about-typhoid/
Study Design, Period, and Area
A retrospective cross-sectional study was conducted on Widal test results registered from December 2019 to June 2021 at Debre Birhan University student clinic. Debre Birhan University is found at Debre Birhan town, North Showa Zone, Amhara National Regional State, which is 130 km North-East of Addis Ababa, the capital city of Ethiopia. A total of 3382 patients were attended at the student clinic during the study period. Of these, 2314 patients who underwent for Widal test were included in the study. After official permission was obtained from the head of the student clinic, the data were retrieved from medical records (registry books) of the laboratory unit for the study period. Cases with Widal test reactive and nonreactive were identified through a review of patient medical records. The data were gathered and saved in excel sheets which were further analyzed by statistical package for the social sciences (SPSS) software version 20 (Figures 2 & 3).
Source and Study Population
According to 2021 Registrar Office reports on July 28, the University has 25,346 students, ten colleges, and 53 departments. Among 25,346 students, 10,759 attended regular programs, 11,890 were summer program students, and 2697 were extension program students. Out of 10,759 regular students, 10,195 were undergraduates (6,469 males and 3,726 females), 547 were MSc students (352 males and 195 females), and 17 were Ph.D. students (15 males and two females). Of 11,890 summer students, 9,826 were undergraduates (7,111 males and 356 females), 2064 were MSc students (1708 males and 356 females). Out of 2697 extension program students, 2,151 were undergraduates (1,195 males and 956 females), 546 were MSc students (409 males and 137 females). The source population of this study was all undergraduate students at Debre Berhan University, and the study population was students who attended the student clinic for the Widal test from December 2019 to June 2021.
Data Collection Tool
The checklist was utilized to acquire data on the Widal test, sex, age, and the department that requested the Widal test.
Quality Control
To ensure the consistency of the data, the necessary data were obtained from the laboratory logbook and reviewed thoroughly every day for completeness and accuracy. Software data have been tried cautiously for mistakes, implausible values, and inconsistencies that might be because of encoding, information, composing, and different bloopers.
Statistical Analysis
The data were gathered and saved in excel sheets, then entered and analyzed using statistical package for social science software version 20. For bar graphs, we were used Graph Pad Prism Software Version 6.
Among 3,382 patients who attended the student clinic, 2225 (65.78%) were males, and 1157 (34.21%) were females. As per (Figure 4), the age of students ranged from 18 to 26 years (mean age was 22, SD = 1.72). Out of 3,382 patients, 75 were 18 years old (45 males and 30 females), 97 were 19 years old (59 males and 38 females), 1677 were 20 years old (1027 males and 650 females), 988 were 21 years old (670 males and 318 females), 356 were 22 years old (318 males and 38 females), 60 were 23 years old (42 males and 18 females), 55 were 24 years old (46 males and nine females), 41 were 25 years old (35 male and six females), and 33 were 26 years old (29 males and four females). Of 3382 patients, 2314 (68.42%) were underwent for Widal test; the rest, 1068 (31.57%), underwent other tests (M ± SD = 1691 ± 881.06) (Figure 3). Of 2314 patients who underwent for Widal test, 1300 (56.17%) were males, and 1014 (43.82%) were females (M ± SD = 1157 ± 202.23). Out of 2314 Widal tests, 1869 (80.76%) were done by blood sample (serum), and the rest 445 (19.23%) were done by a stool sample (M ± SD = 1157 ± 1006.92).
Widal Test
For the total of 2314 febrile patients involved in the study, direct qualitative slide agglutination Widal tests were done using febrile antigen kits of Salmonella typhi (Chromatest Febrile Antigens kits, linear chemicals, Spain). In brief, the test was done by mixing one drop of serum with one drop each of O and H antigens separately on the slide. After shaking the slide back and forth for 1 minute, the mixture was observed for macroscopic agglutination. If there was agglutination within 1 minute, it was reported as reactive; otherwise, non- reactive. Widal tests were done by laboratory professionals who were blind to the study and were based on the manufacturer’s guidelines, and results were given to doctors who requested the tests for patient management (Figure 4).
Prevalence of Enteric Fever/
Of 2314 widal tests done for febrile patients, 890 (38.46%) were reactive for salmonella typhi somatic Lipopolysaccharides O antigen (571 males and 319 females , Chi-square (X2) = 7.5, M ± SD = 445 ± 178.19), 366 (15.81%) were non-reactive for salmonella typhi somatic Lipopolysaccharides O antigen (231 males and 135 females, M ± SD = 183 ± 67.88) , 712 (30.77%) were reactive for salmonella typhi flagellar H antigen (427 males and 285 females, Chi-square (X2) = 7.5, M ± SD = 356 ± 100.41) and 346 (14.95%) were non-reactive for salmonella typhi flagellar H antigen (71 males and 275 females, M ± SD = 173 ± 144.25) (Figure 5). The results indicate that there is no association between widal positivity and gender.
Out of 2314 febrile patients, 1602 (69.23%) were reactive for both O and H antigens. In this study, the test result of slide agglutination for salmonella typhi O antigen is more prevalent than Salmonella typhi H antigen. Our study revealed the seroprevalence of typhoid fever was 69.23%, (95% confidence interval [CI] = 195.58-605.42%). Widal tests done by stool sample were nonreactive for salmonella typhi O and H antigen. Among 1300 males (56.17%), 998 (76.77%) were reactive for O and H antigens (571 for O- antigen, and 427 for H-antigen, M ± SD = 499 ± 101.82), 302 (23.23%) were non-reactive for O and H antigens (231 for O-antigen and 71 for H-antigen, M ± SD = 151 ± 113.14). Of 1014 (43.82%) females, 604 (59.57%) were reactive for O and H antigens (319 for O-antigen (95% and 285 for H-antigen, M ± SD = 302 ± 24.04), 410 (40.43%) were non-reactive for O and H antigens (135 for O- antigen and 275 for H-antigen, M ± SD = 205 ± 98.99) (Table 1 & Figures 5 & 6).
Enteric fever is a dangerous foundational disease happening in non-industrialized nations and keeps on being a significant public health concern. However, authoritative analysis of typhoid fever is by segregation of the bacteria from blood, bone marrow, or other body fluids; most non- industrialized countries like Ethiopia, because of limited laboratory facilities, utilize the old Widal test [15]. Widal test has been utilized in the finding of typhoid fever, since a long in Ethiopia, yet it has low sensitivity and specificity [16]. Our examination showed a 69.23% Widal test positivity rate in a sample of 2314 suspected patients of enteric fever from December 2019 to June 2021 at Debre Birhan University student clinic. The results obtained are comparable with Widal reactive rates of 79.6%, 45.7%, 57.8%,73.9%, 62.3%, 62%, and 44.3% in Delhi, Mysore, Varanasi hospitals, Akoko Nigeria, Sierra Leone, West Gojam-Ethiopia, and Ibadan-Nigeria, separately among the clinically presumed typhoid fever cases [17-23], and furthermore in West Wollega [24] and Ambo [25], Ethiopia, which revealed 53.6% and 56.2%, Salmonella Typhi infection, correspondingly. These variations may be because of study population differences as far as knowledge, attitude, and practice towards enteric fever.
In our investigation, the outcomes of Widal test reactive rates (69.23%) were much higher than with research done from a rural hospital in Maharashtra, India (14.4%) [26], in Bahir Dar, Ethiopia (10.3%) [27], Zaria, Nigeria (14.3%) [28], studies performed in Addis Ababa, Ethiopia (4.1%) [29], Gondar, Ethiopia (4.2%) [30], Ghana (1.7% and 2.4%) [31], Nepal (8.9%) [32], the report from India (14.3%) [33], Bangladesh (30.7%), [34], Ebony, Nigeria,13.2% [35], Enugu, Nigeria, 22.2% [36], Kaduna State, Nigeria, 27% [37], Sokoto, Nigeria, 17% [38], Benin 5% [39], and Kenya (6.4%) [40].
“The difference might be attributed to socio-demographic characteristics, environmental sanitation, water supply, level of drug resistance, sample size, study period, method of laboratory examination, and inclusion of study population.” For example, Dagnew, et al. [30] incorporated all febrile patients without clinical evaluation for the plausibility of typhoid fever, this may make the general prevalence of enteric fever lower in his investigation. In a worldwide setting as well, just one case-control concentrate from Pakistan showed Widal test positivity rate was smaller (11.45%) among 733 clinically speculated patients [41]. Investigations from Vietnam and Nigeria announced the outcomes of Widal test positivity rates were lower, (30.8%) and (24.5%), contrasted with our findings of clinically presumed patients [42, 43]. Our examination outcomes uncovered a higher seroprevalence for Salmonella Typhi infection among guys than females; this might be a direct result of most of the study participants being males. A contrast finding was stated by a cross-sectional study in Northwest Ethiopia where, enteric fever was higher, however insignificant in females (22.5%) than guys (16.7%) [44]. Like our study, an investigation in the profoundly endemic district of Dhaka proposed that typhoid fever is more common in guys than females [45]. Nonindustrialized nations like Ethiopia depend vigorously on widal testing for the determination of typhoid fever. Besides, it has been recognized that blood culture can’t reliably catch Salmonella typhi in all cases [46,47].
The examination is additional confirmation that regardless of its low demonstrative worth, single slide widal tests are being conducted in large volumes in the country. Indeed, we can securely accept that because of the absence of facilities and lengthier turnaround times for the more accurate blood culture; we kept on utilizing the slide Widal test as the single diagnostic test for typhoid fever [18,48]. We frantically need a good point-of-care test for typhoid fever [49]. Since salmonellae are obligate human parasites, the use of the antibacterial drug to treat typhoid fever is generally the pathway through which these bacteria are exposed and develop antibiotic resistance [16]. To sum up, the Widal test in Ethiopia did genuinely well in terms of sensitivity and specificity. However, the test was valuable for eliminating the sickness. Seeing the minimal expense of Widal testing and the shortfall of equivalently inexpensive tests, Widal testing will probably stay the test of choice in many non-industrialized nation settings.
Typhoid fever is a significant public health problem in Ethiopia. Indeed, even today, an enormous number of cases are affirmed through a single semi-quantitative Slide Widal test, despite its known low investigative value. This is prompting bogus judgments and commencement of unseemly drug treatment, which might cause high antibiotic resistance in Salmonella. It is difficult to change this solidly rooted, ancient clinical practice sooner rather than later. As an alternative, we could extricate additional vital data from this continuous practice by working on our current testing and record upkeep systems in small and advanced diagnostic laboratories.
Fundamentally, we comprehend the study of disease transmission of typhoid fever in Ethiopia before we can start decreasing its burden. For this, an initial step is to work on the diagnostic precision of frequently utilized tests in our country, Ethiopia. A uniform health care system should be set up to keep up with and announce Widal test reports by advanced and nonadvanced laboratories. These reports should be followed by better quality kit-based tests and blood cultures desires. We could accomplish this by including both manufacturers of screening tests, and small and advanced private diagnostic facilities in an orderly way in the nearby and territorial observation systems.
All of the required data will be available upon request to the corresponding author
Written informed consent was obtained from each participant.
All authors contributed equally to the reported work, in the concept and design of the research, execution, data collection, analysis, and interpretation and engaged in the drafting and critical review of the paper.
The authors are grateful to thank participating students and
those individuals who gave help directly or indirectly.
Financial support and sponsorship
There is no financial support and sponsorship.
There are no conflicts of interest.


