Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Dimosthenis Chochlakis1,2*, George Dalekos3, Emmanouil Yachnakis4, Eirini Makridaki2 and Anna Psaroulaki1,2
Received: November 01, 2022; Published: November 07, 2022
*Corresponding author: Dimosthenis Chochlakis, Regional Laboratory of Public Health of Crete, Laboratory of Clinical Microbiology and Microbial Pathogenesis, School of Medicine, Crete, Greece
DOI: 10.26717/BJSTR.2022.47.007442
Until recently two rickettsial agents (Rickettsia conorii and R. typhi) were systematically tested in human samples arriving at the Hellenic reference centre. The detection of other Spotted Fever Group Rickettsiae (SFGR) urged towards the implementation of a protocol that tests for five SFGR (R. conorii, R. mongolotimonae, R. slovaca, R. felis and R. massiliae). Environmental data on humidity, temperature, rainfall, and wind were also collected. The current study focuses on the recording of rickettsial diseases in the Greek territory during the last 15 years and attempts an approach on the potential impact of climate on the disease presenta-tion/development. Of the total of 8512 samples investigated, 811 (9.5%) were tested positive for Rickettsia spp (72.3% R. conorii infections, 0.5% R. slovaca, 0.85% R. mongolotimonae, 0.37% R. felis and 0.1% R. massiliae). No clear-cut association between climate alterations and Rickettsial infections was found. IFA remains the routine screening method of choice for many laboratories. Physicians must suspect a rickettsial infection not only in patients with rash, fever, eschar, and lymphadenopathy, but also in those with an adequate epidemiological context. Climate changes may contribute to the emergence of Rickettsioses; however, it is still difficult to model this potential impact.
Keywords: Spotted Fever Group Rickettsiae; Climate; Greece
Abbrevations: SFGR: Spotted Fever Group Rickettsiae; TG: Typhus Group; RLPH: Regional Laboratory of Public Health; IFA: indirect immunofluroescent antibody; HPA: Health Protection Agency; ESR: Eryth-Rocyte Sendimentation Rate; CRP: C-Reactive Protein; MSF: Mediterranean spotted fever
Rickettsioses (caused by strictly intracellular gram-negative bacteria) are typically classified into the typhus group (TG), which includes the agent of the louse-borne epidemic typhus, R. prowazekii, the agent of the flea-borne murine typhus, R. typhi, and the spotted fever group (SFGR) consisting of more than 20 pathogenic Rickettsiae (R. rickettsii, R. conorii, R. mongolotimonae, R. australis, R. akari, R. japonica, R. africae, R. honei, R. slovaca, R. felis, R. heilongjangensis, R. aeschlimannii, R. parkeri, R. massiliae, R. monacensis, R. raoultii, R. helvetica, Rickettsia 364D, R. rioja) [1]. Nevertheless, There are still many more Rickettsiae with a Candidatus status or which are partly or insufficiently characterized. Some authors rise this number to >150, however, this number may not reflect to reality since it is unclear how many will meet current species definitions or are different agents. In general, the classification of many species remains opaque [2]. Typically, Rickettsiae cause a febrile exanthematous illness, such as spotted fever, epidemic, and murine typhus. Humans are incidental hosts of Rickettsiae with the clinical syndrome beginning 6-10 days following an ectoparasitebite. When transmitted to a susceptible human, the pathogenic Rickettsiae multiply in endothelial cells causing a vasculitis, which, in some cases, may result into eschar formation, depending on the rickettsial species. What follows is infiltration of cells adjacent to the vascular lesions (host defense), hemorrhage, and thrombosis due to the degeneration of endothelial capillary cells caused by the growth of the pathogens [3].
Lipopolysaccharides on the outer membranes of Rickettsiae are also thought to participate in the eruption and pyrexia, while endotoxin shock may be observed during the dis-ease leading to the clinical and laboratory manifestations of rickettsial infections. The clinical symptoms are diverse including fever, headache, muscle pain, arthralgias, rash, abdominal pain, vomiting, diarrhea, cough, local lymphadenopathy, and a characteristic inoculation eschar (‘tâche noire’) at the site of the bite. However, these major clinical signs, such as rash appearance, may vary depending on each rickettsial species involved. Nonspecific haematological and biochemical findings include thrombocytopenia, leucopenia, elevated levels of eryth-rocyte sendimentation rate (ESR), C-reactive protein (CRP), urea, creatinine, as well as elevated liver functional tests [4]. The vectors for SFGR are usually ticks (except for R. akari and R. felis, which are transmitted by mites and fleas, respectively). The bacteria are maintained in their reservoirs through transtadial (from larva to nymph to adult) and transovarial (from one generation to another through ovaries) transmission. Each Rickettsia species is transmitted by one or more vectors, therefore the geographical distribution, seasonal activity, host-seeking behaviour or tendency of these arthropods to bite humans play a crucial role in the epidemiology of the disease [5]. Among other factors, climate changes may contribute to the emergence of other rickettsioses or change their distribution and to the dispersal of vectors, their adaptation to new environments or even the hatching of their eggs. Until early 2000, Mediterranean spotted fever (MSF) was considered to be the only rickettsiosis prevalent in Europe. The disease, also known as ‘boutonneuse’ fever, is caused by R. conorii and is transmitted by the brown dog tick Rhipicephalus sanguineus. During the last decade a number of Rickettsia species were detected in ticks and/or in humans, such as R. mongolotimonae (causing lymphangitis-associated rickettsioses - LAR), R. slovaca, R. raoultii, R. rioja (the latter three causing what is known as tick-borne lymphadenopathy - TIBOLA), R. helvetica, R. massiliae, R. monacensis, R. aeschlimannii (the latter three considered as etiological agents of MSF-like illness), together with several Rickettsia species that have been detected in ticks but have not been implicated in human disease yet such as Candidatus Rickettsia kotlanii, Candidatus Rickettsia barbariae, Candidatus Rickettsia vini, Candidatus Rickettsia tselenti, etc [6].
Since symptoms of rickettsial infections are not usually specific, the diagnosis is mainly based on clinical suspicion and serologic analysis followed by amplification of the DNA of the pathogen or even isolation of the pathogen itself. According to the workflow that we follow, DNA amplification is performed in the case of a strong suspicion of rickettsia infection in the absence of any antibodies and/or when antibiotics, that could negatively influence the PCR amplification, have not been administered to the patient for a long time. Therefore, the true prevalence of the disease largely depends on the attention of the clinicians and the quality of the diagnostic tests. The National Reference Center for tick-borne pathogens in Greece was created in 2001 and a preliminary database was kept since then. Since 2010, the database has also included the clinical signs and symptoms along with the laboratory results for each patient tested. Samples were sent to the laboratory under the diagnosis of fever of unknown origin, with or without the presence of rash and/or eschar, and/or living in rural or semi-rural areas, and/or the presence of non-specific symptoms such as myalgia, arthralgia, headache, etc. The current study targets on the recording of rickettsial diseases in a number of areas of the Greek territory (prefecture of Thessaly, Northern Macedonia, islands of Crete and Corfu, prefecture of Attica) during the last 15 years; focusing deeper on the island of Crete, an attempt was made to search for possible connection between the disease and climatic conditions.
Patients
Since January 2001 until July 2014, 8512 samples were received at the National Reference Centre and for SFGR testing on a routine basis. All samples were sent at the Laboratory of Clinical Bacteriology, Parasitology, Zoonoses and Geographical Medicine, University of Crete. In 2010, the Regional Laboratory of Public Health (RLPH) of Crete, branch of the Hellenic CDC, was established and started to collaborate with the laboratory of the University on aspects of surveillance. Samples are generally sent from all Hospitals of the Greek territory but mostly from Hospitals of Crete. When suspicion for rickettsial disease was established at the early stage of the disease and certainly before antibiotic administration, serum samples were accompanied by whole blood samples, as well. In exceptional cases, eschar was also included for testing. Sera were transported at 4oC and always following centrifugation, whereas whole blood samples were transferred at -20oC. Sera were tested by an indirect immunefluroescent antibody (IFA) test, and whole blood samples were subjected to PCR amplification. In case of a positive sample, whether that was serum or whole blood, a follow-up serological investigation was suggested at 21 days. For the purposes of month, seasonal and annual distribution of SFGR, only the result of the first sample was used. A database was created at the beginning of 2000 where data on geographic origin, sex and test results were added. Following the establishment of RLPH, the database was updated in 2010, and since then, detailed data on each patient’s laboratory results, clinical signs and symptoms are kept. In particular, a clinical- epidemiological record forma was created, which was filled in by the clinician and accompanied each sample. The record forma was created after communication with the Health Protection Agency (HPA) of the UK, where similar records are being filled in by the clinicians.
Environmental Data
Data on air temperature, wind direction, mean relative humidity and rainfall were collected from the Hellenic National Meteorological Service and from the National Observatory of Athens. Data were made available since 2006 onwards, hence the processing of data and distribution maps from this date on. Data corresponded to the island of Crete only; the study of a possible relationship between climatic data and human cases was focused on this area since most cases resided from there. Data on vectors, their abundance and distribution were not included in the current study.
Serological Analysis
From 2001-2009 all samples received were tested for R. conorii; from 2010 and onwards, all samples are routinely checked for antibodies against Rickettsia spp using a slide that could test against R. conorii, R. mongolotimonae, R. slovaca, R. felis and R. massiliae as individual antigens (Fuller laboratories, California, USA). Sera were tested at dilutions beginning from 1/25 and from 1/60 in respect to immunoglobulins (Ig)G and M antibodies, respectively. Single sera at IgG titers of ≥1/960 and/or IgM titers of ≥ 1/200 and sera with seroconversion (four-fold increase) between acute and convalescent phase were considered positive. The choice of the cut-off was based on past studies carried out on blood donors not only for rickettsia species (data not shown) but for other pathogens as well. [7-9] To avoid false positive results and to exclude as much as possible cross-reactions, the cut-offs were selected following a survey on SFGR on blood donors in Crete, where the actual seroprevalence among otherwise healthy population was high (unpublished data). In any case, samples were considered as matching to true rickettsial infection only after confirmation by the corresponding clinician.
Molecular Analysis
DNA was extracted from the whole blood samples received by using the QIAamp DNA blood mini Kit (QIAGEN, Hilden, Germany) and from eschars by using the DNeasy Tissue kit (QIAGEN, Hilden, Germany) according to the manufacturer’s instructions. Until 2010, samples were PCR amplified targeting a 381 bp portion of the citrate synthase gltA gene [10]; in cases of a positive result samples were further amplified targeting a 532 bp portion of the ompA gene [11]. Since 2010 the Real-time PCR was introduced targeting the gltA gene of Rickettsia spp [12]. All positive PCR prod-ucts were purified (PCR product purification kit; QIAGEN, Hilden, Germany) and were directly sequenced. Nucleic acid sequences were processed using nucleotide BLASTn, Chromas v1.49, and Lasergene Version 7.1 software.
Statistical analysis
The time series data variables of this study (N=96, corresponding to 8 years or 8x12=96 months) were the number of cases, the temperature, the humidity, the rainfall level and the wind direction. All statistical analyses were conducted using the IBM SPSS Statistics Version 21 statistical package. Statistical significance was set at p = 0.05 (two-tailed Ps <0.05 were considered statistically significant). Mainly: (a) Descriptive analysis was used to describe the basic features of the data, for each variable individually, (b) Multiple linear regression analysis was performed for investigating if and how the variable number of cases depends on the rest three quantitative variables (temperature, humidity, rainfall level) by means of a liner regression model or, in other words, to find if such a meaningful model exists, and which of these variables contribute meaningfully to the model. The variables were, also, analyzed by means of the Fourier transform for accessing any relevant frequencies. This kind of transformation was applied because it represents the best balance be-tween a low number of explanatory variables with an adequate description of the climate traits [13]. Phase portraits were produced for visualizing their underlying dynamics of the variables. In dynamic systems each set of initial conditions is represented by a different curve, or point.
Clinical/Laboratory Data
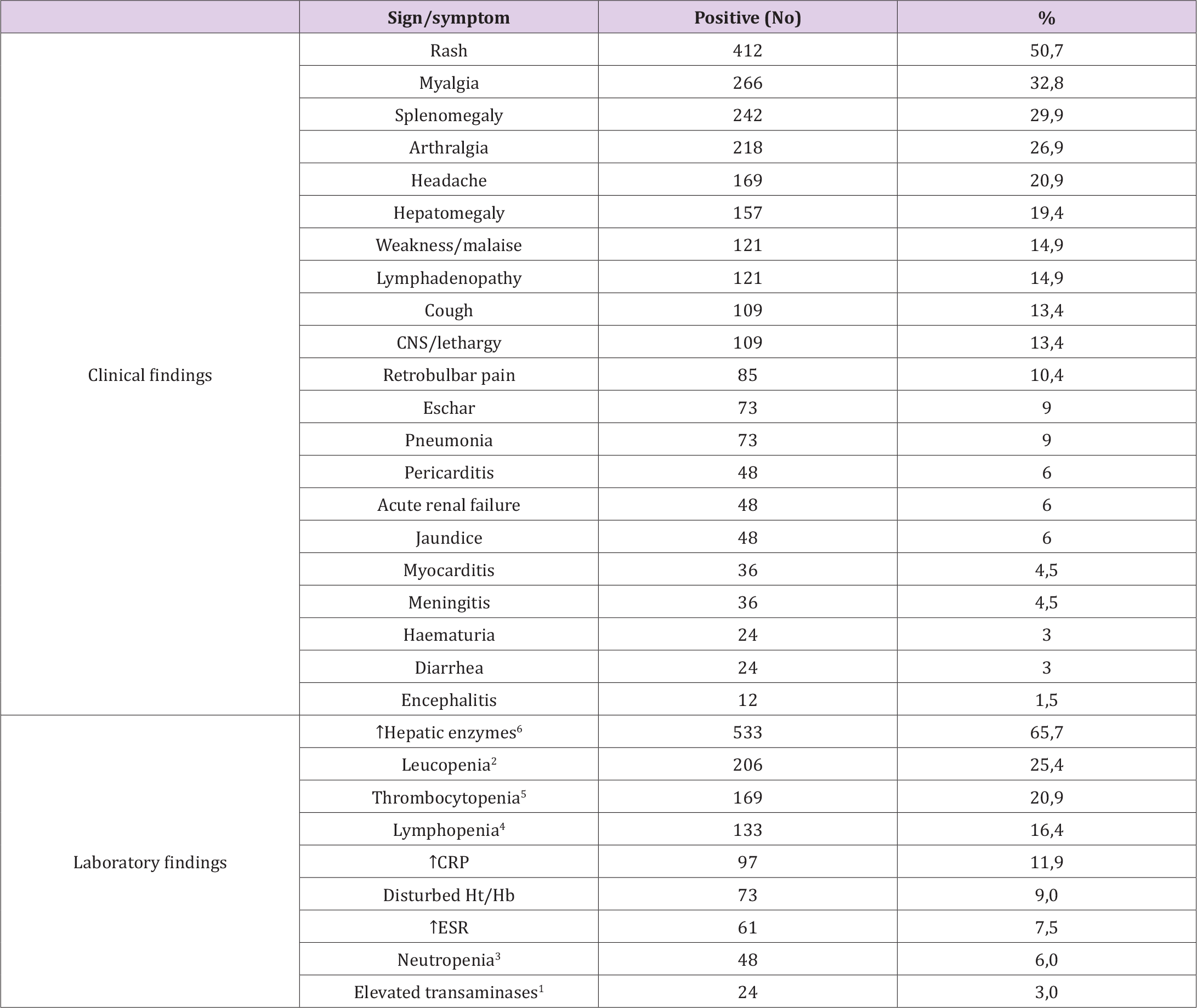
Eight hundred and eleven (811) out of 8512 tested patients (9.5%) were tested positive for Rickettsia spp by IFA. A second sample was received in 215/811 (26.5%) of positive samples (33 at 15-21 days, 101 at less than 15 days and 81 in more than 21 days). A third sample was received in 48/215, a fourth sample in 11/48, a fifth in 6/11 and a sixth in five out of six. Rickettsia conorii infections were identified in most cases (586/811, 72.3%) followed by R. typhi (210/811; 25.9%). In addition, 15 samples were tested positive for SFGR and in particular: four samples were tested positive for R. slovaca (0.5%), seven for R. mongolotimonae (0.9%), three for R. felis (0.4%) and one for R. massiliae (0.1%). The diagnosis of the aetiological agent was determined without serum cross-adsorption since in these 15 cases the antibodies against the aetiological agent differed by at least four-fold from the rest of the rickettsial agents tested. Eighty-six (86, 10.6%) samples were tested positive based on IgG only, 418 (51.5%) on IgM only and 307 (37.9%) on both IgG and IgM. Eight whole blood samples were tested positive by Real-time PCR without antibody titers on their accompanying sera. Eleven samples fulfilling epidemiological (tick-bite, occupation with animals of veterinary importance) and clinical (fever, rash, eschar, increased transaminases) signs revealed no antibodies against the tested Rickettsia species. All 811 patients had fever (range 38.3 – 40.2oC at the time of sampling). A large distribution of signs and symptoms, most of them being non-specific, was recorded, as presented in (Tables 1 & 2).
The discussion examined a health pandemic affecting a growing number of individuals in the United States. Health problems related to a needs assessment included baseline data collected from research journal articles and other health related agencies. The health pandemic was prioritized by identifying target populations in the United States who have been affected by autoimmune diseases, vaccine damage, and high levels of inflammation [1-5]. A needs assessment can be administered to target populations via small groups, in-person, in-clinic, or online consultations as well as online curriculum modules. Furthermore, the discussion explained program objectives and goals for a health education and intervention program for autoimmune disease patients. In addition, a comprehensive project management intervention strategy and implementation plan was summarized. How to monitor and evaluate the intervention plan was described. Finally, the role of program evaluators was addressed. Eleven graphics were included throughout the document.
Table 1. Signs and symptoms of patients suffering from a rickettsial infection. The 811 patients for whom we had partial and/or full clinical and laboratory data, are included. 1: Normal values (NV) 5-40 IU/ml; elevated values >3 x NV. 2: <4000 leucocytes/μl. 3: <1000 neutrophils/mm3. 4: <1500 lymphocytes/μl. 5: <150,000 thrombocytes/μl. 6: elevated val-ues >3 x NV.
Environmental associations
A peak in positive samples was recorded during 2005 with the increase beginning from 2001, followed by a continuous drop to the lowest numbers in 2008, when positive samples started to increase again (Figure 1). Considering seasonality, most cases appeared to peak during autumn and then gradually drop, except for 2008 winter, 2012 and 2013 spring, in which increased human cases were also recorded (Figure 1). Generally, an increase in the number of cases was observed in the presence of north winds and/or its variations such as north-west and north-east winds. An exception was observed during the years 2012 and 2013 where slightly increased cases were recorded even in the absence of north winds (Figure 2). In regard with temperature, cases seemed to increase in number during late summer and early autumn and almost al-ways when the temperature started to decrease. An exception was observed during the winter of 2005 – spring of 2006 and winter of 2011 – spring 2012 where a slight peak of cases was also recorded. However, an increase in temperatures did not generally seem to favor the appearance of cases (Figure 1).
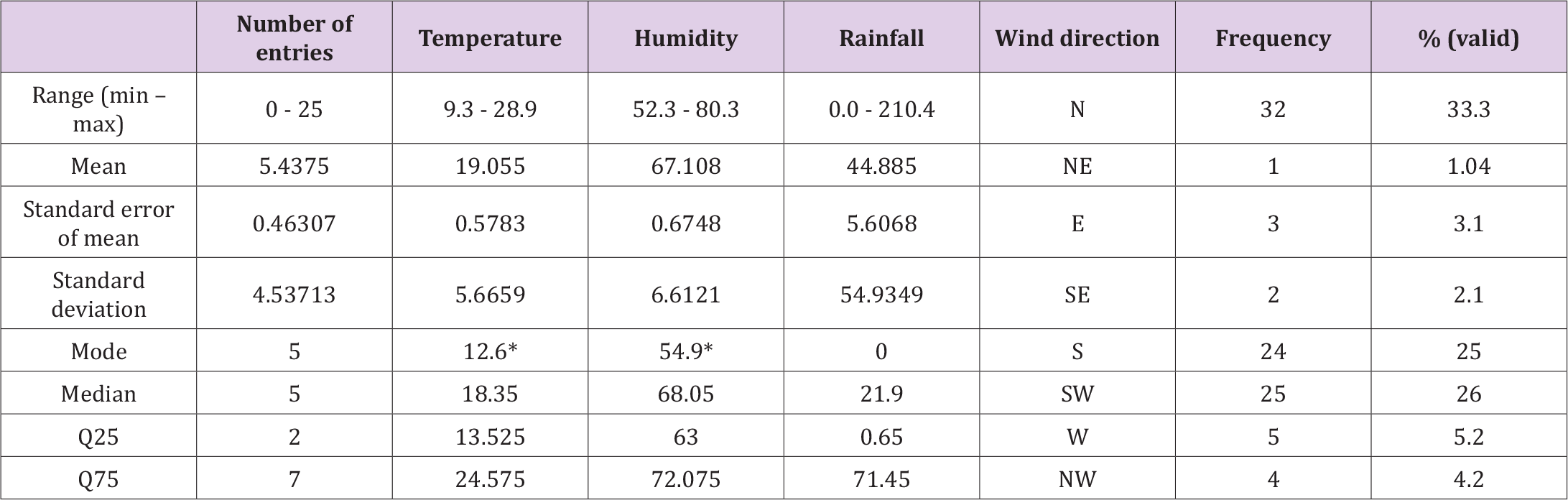
Furthermore, concerning rainfall, the presence of increased human cases as described above coincided with the start of slight rains, with the exceptions being the periods described above (Figure 1). Regarding humidity, most human cases were commonly described during decreased levels, either during its dropping or just before its rising. Nevertheless, the variable number of cases did not associate significantly with anyone of the above three quantitative variables (temperature, humidity, rainfall level), and thus, we were not able to define a meaningful linear regression model describing the dependence of the number of cases on these variables. That was, also, assured by taking the data summed up seasonally. Specific conditions (combinations of the three variables above) reasonably considered as favoring the increase of the number of cases, were not proven here as that, despite the apparently deterministic nature of the complex dynamical system generating the data under consideration (Figure 3, Table 3).
Table 3. Descriptive statistics of the data variables (96 entries – 8 years x 12 months) of the study. *The smallest value of multiple modes.
Tick-borne diseases and rickettsial infections are distributed all over the European territory. The change in climate together with interventions in the environment (deforestation, change of land use, expansion of cities towards the niches of vectors, expansion of cattle breeding towards areas where wild animals breed, change of climate that creates pressure to vectors to migrate to areas that provide more friendly conditions, etc) have led to a spread of tick species to areas where these infections were absent or of low prevalence and incidence in the past. In Europe, there is an increasing tendency to import new tools in the surveillance of tick-borne diseases. The most obvious one is the systematical recording of human cases. However, the achievement of this goal depends on the implementation of a specific surveillance system and on the willing of clinicians to report human cases. A different approach might be the testing of the role of animals as sentinels of pathogen and/or of ectoparasite distribution. Monitoring the circulation of pathogens in wildlife, pets or cattle may provide evidence for the presence of high or not risk in the transmission of pathogens [14]. However, apart from the above approaches which include classical tools of microbiology, there is an increased tendency of incorporating other scientific tools like the use of mathematical modeling and the careful observation of climate changes. Such alternative approaches have already been implemented in the case of tick-borne encephalitis for example in Sweden where climatic, wild-life and human cases of infection were used to export a model that was finally used as an early warning system [15]. In other words, in vector-borne diseases, the information collected is so large that the need to process, study and understand it requires the introduction of multiple approaches such as climatic and mathematical models, classical statistics, and Artificial Neural Networks [16]. Indeed, in our study the increase or decrease in the number of cases recorded did not seem to be directly linked to the quantitative variables tested (temperature, humidity, rainfall level). These environmental variables may affect the inbreeding of vectors which may ultimately lead to more human cases. Changes in the environmental conditions may also favour the introduction of new vector species in an area leading to the same result. These, at first glance, interdependent variables need more sophisticated modelling approaches to reveal their potential relations.
In a seroprevalence study carried out in Tanzania, an attempt was made to associate environmental conditions with the presence of antibodies against Rickettsia species. Indeed, amongst others, the multivariate analysis in that study revealed an association of rickettsioses with warmer temperatures [17]. In fact, the authors suggest that higher temperatures seem to increase host-seeking behavior, breeding and survival in many tick species.
Ticks of the genus Rhipicephalus are the most common with close association between their presence and human cases of rickettsial infection. Indeed, R. sanguineus is well adapted to urban environments and lives in close contact with hu-mans and in Europe the tick starts to be active in May and June [18] while most cases of R. conorii infection are diagnosed during July and August. It has generally been suggested that increased temperature leads to an increased period of activity of R. sanguineus and an increased aggressiveness and tendency to bite humans and mammalian hosts [19]. Indeed, in a study carried out in southern France [19] in 2007, it has been shown that during the exceptionally warm April and May months of 2007 a number of patients suffering from R. conorii and R. massiliae infections were recorded; this finding was attributed to an increased aggressiveness of R. sanguineus due to the higher temperatures. However, it should be noted that the eggs, lar-vae, and unfed nymphs of infected and non-infected ticks cannot tolerate low (4°C) or high (37°C) temperatures or long starvation periods [20], which may partly explain the presence of most cases described in our study during late summer and/or early autumn. The increase in human cases, whether that was less or more obvious, in the presence of north (or north-west or –east winds) observed in our study needs further investigation, since ticks are not carried by winds, how-ever their maturation and distribution might be directly or indirectly related to wind direction. A different study [21] working on Ixodes, Haemaphysalis, Rhipicephalus and Dermacentor has demonstrated a positive relationship between tick abundance and ambient temperature and/or mild winters. It has been proposed that certain ticks such as Dermacentor species, bite more readily during the cool season [22]; this may partly explain the presence of increased numbers in our cases during winter of 2005 – spring of 2006 and winter of 2011 – spring 2012. However, this study was conducted in Poland which has a colder climate compared to Greece. Therefore, the latter finding needs further verification in our country as well. With regards to rainfall, it has already been predicted that decreased rainfall may create conditions suitable for an in-crease in the extent of habitats for R. bursa, R. turanicus, and H. marginatum [23].
Humidity seems to play a key role on the early stages of maturation of the eggs of ticks. In a recent study [24], the authors concluded that successful maturation and eclosion of larvae depended both on high ambient humidity and on tick species. In particular, R. sanguineus, seem to have managed a better adaptation to dry environments compared to other spe-cies; this may partly explain why R. sanguineus occurs less commonly than other tick species in moisture- rich environments. Perhaps this may explain the distribution of most of our human cases around humidity levels of 65%.
As far as clinical manifestations are concerned, a wide range of them have been described in tick-borne rickettsioses throughout Europe. Acute fever is present in all cases, and it is generally accompanied by chills, headache, photophobia, arthralgia, muscular pain, etc. The rash (maculopapular, occasionally with purpuric elements, most frequently found in extremities and rarely in the trunk) usually appears 3-5 days after the onset of fever [25]. In our cases, 11 different rash “types” were recorded, while a large variety of signs and symptoms was also recorded. In Europe, most cases are recorded in the summer perhaps due to higher temperatures, lower rainfall, or perhaps due to a warming-mediated in-crease in the aggressiveness of R. sanguineus ticks to bite humans [6]. Although MSF and DEBONEL/TIBOLA are the most frequent ones, infections due to different Rickettsia species may also occur, as demonstrated in the current study. Diagnostic issues arise when a rash and other typical sign such as eschar are absent. In Greece, six SFGR species have been detected in ticks, i.e., R. conorii [26], R. massiliae [27], R. mongolitimonae [28], R. aes-chlimannii, R. rhipicephali [29] and R. slovaca [30]. Of those, five SFGR species have been implicated in human diseases in the same country, i.e., R. conorii [26,31,32], R. aeschlimannii [33], R. mongolotimonae [28], R. massiliae [34] and R. slovaca [35]. Rickettsia felis has been isolated and/or detected in fleas (Ctenocephalides felis), and three cases have described in humans in the area of Chalkida in Euboea, an island north of Athens [36].
Until 2002 only R. typhi and R. conorii were considered to cause human infection in Greece and were routinely tested by IFA. The description of R. mongolotimonae and R. aeschlimanii in humans and of R. slovaca and R. massiliae in ticks, urged us to set up a protocol testing for another four Rickettsia species under the suspicion that other mis-/under-diagnosed cases existed. Based on the results of the current study, in the absence of this protocol, 15 human cases would have been missed. Of course since the protocol is largely based on the amplification of gltA as an initial screening method and on the application of antibody testing, it is obvious that no confident identification of the exact rickettsia species can be made. If the exact species is need for epidemiological and/or other reasons, the only certain way is the amplification of other genes such as the ompA. Since signs and symptoms are not generally specific, rickettsial infection does not constitute the first diagnosis of choice for most clinicians. Thereafter, samples (sera and whole blood) are tested days after the onset of symptoms and, in most cases, following the administration of antibiotic therapy, minimizing therefore the pos-sibility to detect a positive PCR, while the detection antibodies of acute infection may be influenced as well depending on the antibiotic regimen, the number of days under antibiotic administration, etc. Serological methods may lag PCR amplification and culture however, their implementation may still solve problems and give answers. In any case, each method acts in a complementary way with the others to provide the best possible laboratory diagnosis.
Limitations of the Study
The results of the current study were largely based on the detection of antibodies therefore no definite laboratory diagnosis on the exact species could be made. Since a rickettsia infection does not produce specific symptoms, most samples were received post antibiotic administration minimizing the possibility of detecting a positive PCR. Several patients were not tested at the convalescent phase of their disease largely because clinicians do not tend to test a later sample if the patient resolves, despite our suggestions. No tick data were collected for the period of study, this would require the collaboration with veterinarians which was not made possible, therefore no direct connection on the distribution of the cases throughout the years against vector distribution was possible. Certainly, the proper active surveillance of vector-borne pathogens requires the cooperation of multiple scientific specialties and not a simple recording of human cases. Entomologists, mathematicians should also come into play since the role of the climate is both crucial and difficult to interpret.Six Rickettsia species (R. conorii, R. aeschlimanii, R. slovaca, R. mongolotimonae, R. massiliae, and R. typhi) have been de-scribed in Greece, either in ticks and/or humans; perhaps many more are out there. Physicians should keep in mind a clinical suspicion for index cases with unexplained fever even if the typical manifestations of rickettsioses are missing and always take under consideration the epidemiological context, particularly in endemic areas of the disease. Climatic conditions may, under certain circumstances, indirectly affect the presence and prevalence of rickettsial infections in a certain area. Though the application of a prediction model seems fascinating, several critical factors of minor or major importance like presence of ticks, or their hosts, etc seem to affect the presence of the disease, making the construction of a robust model a little more complicated than anticipated. We did not attempt any correlation between presentation of specific clinical signs and symptoms against climatic conditions; however, this could be part a future study.
Conceptualization, Dimosthenis Chochlakis and Anna Psaroulaki; methodology, Dimosthenis Chochlakis and Eirini Makridaki; software, Emmanouil Yachnakis; formal analysis, Dimosthenis Chochlakis and Emmanouil Yachnakis; data curation, Emmanouil Yachnakis and George Dalekos; writing—original draft preparation, Dimosthenis Chochlakis; writ-ing—review and editing, George Dalekos and Anna Psaroulaki; supervision, Anna Psaroulaki. All authors have read and agreed to the published version of the manuscript.”
This research received no external funding.
Informed consent was obtained from all subjects since all clinical- epidemiological record forma were signed by the corresponding patients who gave their permission for their samples to be used for research purposes.
We would like to thank all clinicians at all Hospital Units from which we receive samples, for their excellent collaboration without which, the creation of the current manuscript and the gather of all the knowledge on rickettsial infections, would have been impossible.
The authors declare no conflict of interest.


