Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Cheang Agnes1*, Nabila Perveen1, Syed Atif Raza2 and Naeem Hasan Khan1
Received: September 15, 2022; Published: September 27, 2022
*Corresponding author: Cheang Agnes, Department of Pharmaceutical Chemistry, Faculty of Pharmacy, AIMST University, Kedah D.A., Malaysia
DOI: 10.26717/BJSTR.2022.46.007345
The present study aims to compare the quantitative determination of metformin hydrochloride brands available in Malaysia by spectroscopic method and Fourier transform infrared spectroscopy. Several different commercially available leading brands of metformin, within their shelf-life were purchase from various pharmacy outlets in Sungai Petani, Malaysia. Each brand of metformin hydrochloride tablets was labelled to contain 500 mg of metformin hydrochloride. The metformin tablets were blindly named as Brand A, Brand B, Brand C, Brand D, Brand E, Brand F and G by spectroscopic method and Fourier transform infrared spectroscopy in the present study. The average percentage purity of sample A, B, C, D, E, F and G was 99.15%, 99.03%, 99%, 99.66%, 98.31%, 99.42% and 99.38%. The brand A, B, C, D, F, and G passed the test whereas brand E failed the test according to the United States Pharmacopeia (USP). Several brands of Metformin Hydrochloride being assessed in this research, it showed that most of the brands were within the pharmacopoeia limits for various quality control parameters except one. Through the FTIR spectral measurements, a satisfactory vibrational band assignment of metformin hydrochloride has been carried out.
From the outcome of the various tests, it indicated that the process validation data provides a high degree of assurance of the manufacturing of these tablets’ products meet its predetermined specification and quality attributes.
Keywords: U.V. Spectrophotometer; FTIR; Netformin; U.S. Pharmacopeia; Analysis
Metformin is a class of drugs named biguanides prescribed for type 2 diabetes in the result of debilitated insulin discharge [1]. Treatment decision contains, oral antihyperglycemic and diet / insulin [1]. Metformin is an oral biguanide, enhances hyperglycemia to improve peripheral sensitivity toward insulin and reduces gastrointestinal glucose formation [1]. As sulfonylureas, which doesn’t stimulate secretion of insulin, aggravate hyperinsulinemia or because hypoglycemia or gain of weight (weight balances or reduces). It has also useful effects on serum lipid profiles. Metformin is sheltered and successful equally as monotherapy and mix through antihyperglycemic specialists to sort diabetic 2 patients ̓ needful extra glycemic control might be advantage when control of weight is desirable [1]. Metformin is currently a firstline treatment for Type 2 diabetes [2-5]. It is not insulin but is considered as insulin sensitizer [6,7]. It is in the biguanide family, isolated from the flower Galega officinalis, commonly known galega or goat’s-rue and was used as treatment for diabetes in the medieval period because it relieved the intense urination [8]. The guanidine structure of metformin can be thought of as a nitrogenous analog of carbonic acid and includes two methyl groups, whereas phenformin and buformin have apparently toxic aromatic rings or alkyl chains.
In Figure 1, the differences between phenformin and metformin can be observed. The botanical Galega officinalis, previously mentioned, was given to diabetic patients because it relieved the excessive urination symptom. G. officinalis, also known as either with the name of French lilac or Italian fitch was also given as a medicine during the plague epidemics to perspiration [8]. In the French lilac, there is an active ingredient that can lower the blood glucose level and the ingredients was shown to be galegine or isoamylene guanidine (Hakeem-Habeeb, B.,2011). Metformin’s official chemical name is N, N-dimethylimidodicarbonimidic diamide (also called 1, 1-dimethylbiguanide) or C4H11N5 [9]. In 1922, metformin was described firstly by Werner and Bell, and it involved a precipitation reaction of dimethylamine hydrochloride and 2-cyanoguanidine over heat. This one step synthesis can be seen in Figure 2 [10]. Metformin hydrochloride, the salt version of the drug, is synthesized via the reaction of equimolar quantities of 2-cyanoguanidine with dimethylamine and thus made into a solution using toluene with the process of cooling, with equimolar amounts of hydrogen chloride slowly added.
The mixture boils and then, after cooling, metformin hydrochloride is yielded in a precipitate with 96% yield [7-11]. The dosage of metformin is usually 1-3 pills per day at different sizes of 500 mg or 850 mg or 1000 mg, taken with meals in order to reduce adverse gastrointestinal side effects [7]. The dosage is usually changed when taken with the combination with other drugs. Metformin can be used concomitantly with other drugs such as glipizide, glyburide, rosiglitazone, pioglitazone, repaglinide, sitagliptin, and insulin to create beneficial glucose lowering effects [12]. According to IUPAC, spectroscopy can be defined as “the study of physical systems by the electromagnetic radiation with which they interact or that they produce. Spectrometry is the measurement of such radiation as a means of obtaining information about the systems and their components [13]. The interpretation of the resulting spectrum can be used to analyze elements and chemical compounds, examine molecular structures, and determine the composition of a material [13]. UV spectrophotometry is another analytical technique which is being widely used in the chemical research for quantitative and qualitative analysis or organic and inorganic compounds.
Generally, UV spectra can be used with confirmation by comparing the spectra of the suspected compound [14]. For determining the overall structure, data from other instruments, such as FTIR, NMR and other supporting data are usually used [15]. This paper can be used as a guide for researchers and novice students in understanding and interpreting UV spectrum data. Fourier Transform Infrared or FTIR is a valuable analytical technique for researchers. This type of analysis is mainly used for characterizing unknown compounds in the forms of solutions, liquids, powders, fibres, pastes, films and gases. It is also possible in analysing the material which is on the surfaces of substrate [16]. Compared to other types of characterization analysis, FTIR is quite popular. This analysis which helps in characterization is good in accuracy, sensitive and also rapid. [17]. During the FTIR analysis, samples will get subjected to contact with IR radiation. The interaction of infrared radiation with molecules in the sample with cause the impact of atomic vibrations of a molecule and then further resulting the specific absorption or transmission of energy. This makes FTIR is quite useful for determining and analyzing the specific molecular vibration in the sample [18].
The concentration determination of each brand of metformin hydrochloride tablets available in Malaysia is comparative with official monograph. According to United State Pharmacopeia (USP), metformin hydrochloride must contain not less than 98.5 percent and not more than 101.0 percent of C4H11N5.HCl, calculated on the dried basis (Figure 3).
Spectroscopic analysis including absorbance measurements was carried out using UV-VIS spectrophotometer Shimadzu UV- 1800 with 1cm path length quartz cells as shown in Figures 4 and 5. It is connected to the analyzed computer for interpretation and determination of absorbance. For weighing, Fisher brandTM analytical balance comprising of large draft shield with sliding top and side doors is used.
Metformin hydrochloride BP powder as standard solution, several brands of metformin (branded and generic version) as sample solution and distilled water as blank solution. They are all of analytical grade.
Several commercially available leading brands of metformin, within their shelf-life were purchased from various pharmacy outlets in Sungai Petani, Malaysia. Each brand of metformin hydrochloride tablet was labelled to contain 500 mg of metformin hydrochloride. The metformin tablets were blindly named as Brand A, Brand B, Brand C, Brand D, Brand E Brand F and Brand G in the present study. The descriptions about the different brands are shown in Table 1.
Twenty tablets of metformin hydrochloride were weighed and then powdered using mortar and pestle. A quantity of the powder equivalent to 0.1 g of metformin hydrochloride was made into solution by adding distilled water, the resulting solution was then diluted with distilled water and the absorbance of the resulting solution was measured using UV- visible spectrophotometer at a wavelength 233 nm. The content of metformin hydrochloride was then calculated using calibration curve. The experimental procedure was then repeated for the other brands.
The Lambert-Beer law allows for the determination of the sample concentration from the measured absorbance value. If the extinction coefficient ε and the path length d are known, then concentration c can be calculated from absorbance A as given below:
• c= concentration (in mol/L or g/mL)
• d= path length (in cm)
• ε (epsilon) = special constant describing how much the sample is absorbing at a given wavelength (in L/(cm*mol) or mL/(cm*g)
When the path length is 1 cm and the concentration is 1% w/v, the extinction coefficient is called specific absorbance
Based on the Lambert-Beer Law, the concentration of a compound in a solution can be determined quantitatively by UV/VIS spectroscopy. To perform that, a calibration line is first determined by measuring the absorption of several standard solutions of known concentration. In this way, the concentration of samples such as, DNA, RNA, proteins, carbohydrates, organic compounds can be determined.
A calibration curve of standard metformin hydrochloride tablet was drawn. The relationship between concentration and absorbance was plotted and the equation and correlation values of the curve were generated from the scatter plot.
USP is used as one of the standards for the evaluation study. The concentration of several metformin HCl samples were compared with standard to determine whether the sample comply with the official limits. The samples were also compared with each other to determine whether which one is better than others [19].
Tables 2A-2G: Weight uniformity of different brands of Metformin hydrochloride.
At different intensities and locations of the IR spectrum, there are different functional groups which can produce bond absorptions. When we get to recognize the absorptions generated by the common functional groups occur, this will help in interpreting IR spectra. The Table 2 below shows the list of the intensities and the locations of the absorption being produced by typical functional groups [20].
Metformin Hydrochloride was white crystalline powder, odorless and has bitter taste. For the solubility test, metformin hydrochloride is freely soluble in water, and it is slightly soluble in alcohol, and it is practically insoluble in acetone and methylene chloride. Also, the pH value of 1% aqueous solution of metformin hydrochloride is 6.68.
The average weight of different brands of metformin hydrochloride tables are shown in Tables 2a-2g. These tables shows that the Glucovance had the highest average weight 629.5 per tablet while Metformin had the lowest average weight 525.5 per tablet. The average weight for Glumet DC were 533.8 mg, Glucoxit Duopharma 540.6 mg, Metformin HCl Sunward 601.6 g, Glucophage 530.5 mg per tablet and Diamide Film Tablet 617 mg per tablet respectively. The purpose of this test is to verify the uniformity of each batch that reflect the drug content uniformity in all the formulation batches. This test was performed as the official procedure. Furthermore, the different weights of the tablet may differ in the quality parameters. This includes the content of the active pharmaceutical ingredient. The average weight of the tablets of the seven brand ranges from 520 to 620 mg.
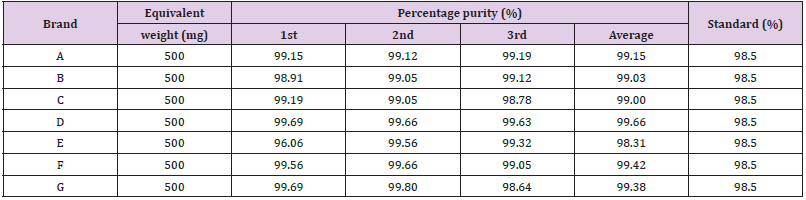
Absorbance of different brands of Metformin Hydrochloride using distilled water as solvent is laid down in Table 3 and Graphs 1-7 shows the UV absorbance 0f different brands.The UV absorbance of different brands of Metformin Hydrochloride is shown in Table 1. The first, second and third readings of standard Metformin Hydrochloride were 2.948, 2.947 and 2.946. In the trial, the first, second and third readings were 2.923, 2.921 and 2.922. The average for trial is 2.922 and the average for standard is 2.947.The first, second and third readings of standard metformin hydrochloride were 2.947, 2.946 and 2.945 and the average for the standard was 2.946. There was trial conducted for assay of brand B and the trial has 3 readings. The first, second and third readings were 2.915, 2.918 and 2.919. The average for the trial was 2.917.The first, second and third readings of standard metformin hydrochloride were 2.945, 2.947 and 2.946. The average standard reading was 2.946. There was trial conducted for assay of brand C and the trial had 3 readings. The first, second and third readings were 2.921, 2.919 and 2.91. The average for the trials was 2.917. The first, second and third readings of standard metformin hydrochloride were 2.94, 2.944 and 2.946. The average for the standards was 2.943. There was trial conducted for assay of brand D and the trial had 3 readings. The first, second and third readings were 2.931, 2.934 and 2.935. The average for the trials was 2.933. The first, second and third readings of standard metformin hydrochloride were 2.946, 2.947 and 2.943. The average reading for the standards was 2.945. There was trial being conducted for the assay of brand E and the trial had 3 readings. In the trial, the first, second and third readings were 2.83, 2.934 and 2.923. The average reading for the trials was 2.896.The first, second and third readings of standard metformin hydrochloride were 2.941, 2.940 and 2.938. The average reading for the standards was 2.940.
There was trial being conducted for the assay of brand F and the trial had 3 readings. In the trial, the first, second and third readings were 2.928, 2.930 and 2.910. The average reading for the trials was 2.923.The Percentage purity of different brands of Metformin Hydrochloride is shown in Table 4 by UV spectrophotometric method. The first, second and third readings of standard metformin hydrochloride were 2.943, 2.942 and 2.940. The average reading for the standards was 2.942. There was trial being conducted for the assay of brand G and the trial had 3 readings. In the trial, the first, second and third readings were 2.934, 2.936 and 2.90. The average reading for the trials was 2.923. The average percentage purity of sample A, B, C, D, E, F and G was 99.15%, 99.03%, 99%, 99.66%, 98.31%, 99.42% and 99.38%. The brand A, B, C, D, F, and G passed the test whereas brand E failed the test. According to the United States Pharmacopeia (USP), the percentage purity of the Metformin Hydrochloride should be in the range of 98.5% to 101.0%. The comparison of percentage purity of sample with the standard (%) is given in Graph 8. According to the United States Pharmacopoeia (USP), the percentage purity of the Metformin Hydrochloride should be in the range of 98.5% to 101.0%. All of the brands passed when using the distilled water as solvent. To conclude, six of the seven samples which were brand A, B, C, D, , F, and G complied with U.S.P official limits with percentage purity of 99.15%, 99.03%, 99%, 99.66%, 99.42% and 99.38% respectively. For brand E, it failed to comply with the limits and had the percentage purity of 98.31%.
This is most probably due to the hygroscopicity of the metformin hydrochloride. Metformin hydrochloride is high water soluble. It can absorb the atmospheric moisture so rapidly that the concentration of the prepared solution is usually changes over time. This concern is reflecting in the absorbance readings. To eliminate this problem, when preparing the solution for the UV spectrophotometry, it has to be standardized to know the exact concentration and only prepared when needed to keep it as fresh as possible. Once the drug content has been exposed to the atmosphere and become extremely sensitive to the temperature, light and humidity. Although the samples had been stored well in a plastic zipper bag and placed in a desiccator, the result showed the stability of the samples might be slightly affected in the factor of consideration of the tablet sample solution. Factors such as light, temperature and humidity may cause significant drug degradation and thereby affect its label content and purity [21].
Table 4: Percentage purity of different brands of metformin hydrochloride UV spectrophotometric method.
Infrared spectrum gives information about the functional groups present in the compound. The FTIR spectrum for the metformin hydrochloride is recorded and given in Graphs 9-10. The wave number values for each peak have been explained in Table 5. The FTIR spectrum was found to be in compliance with the various peaks for metformin HCl. All the tests of the identification studies were found to be compliance with the data available in the literature. Also, it is confirmed from these results that the procured drug Metformin HCl was free from the impurities. Other than that, background spectrum contains information about the species of gases and solvent molecules, which may be subtracted away from our sample spectrum in order to gain information about just the sample. The reference standard for the metformin hydrochloride during the infra-red (IR) scan is characterize by the wave numbers of 740, 935, 1075, 1063, 1580 and 1620cm-1. And for the N-H wagging vibrations it occurs at 740 and 935 cm-1: C-N stretch vibrations for 1063 and 1075 cm-1 and C=N stretch vibrations accounting for absorption bands at 1580 and 1620 cm-1. The identification test results indicated that the generics of metformin hydrochloride tablets being used for this research as Active Pharmaceutical Ingredient (API). The N-H stretching vibrations are found in the region 3300- 3500cm-1. In the IR spectrum, there is a peak appearing at this region as it is assigned to the N-H stretching vibration. There is C-H stretching vibration generally occur in the region 3500- 3300 cm-1 and in the region of 3200-3000 cm-1. There is C-N stretching vibration generally occur in the region of 1500-1000 cm-1 with the peak at 1416 cm-1.
From the several brands of Metformin Hydrochloride being assessed in this research, it showed that all of them were within the pharmacopoeial limits by looking at the various quality control parameters. Through the FTIR spectral measurements, a satisfactory vibrational band assignment of metformin hydrochloride has been carried out. From the outcome of the various test, it indicated that the process validation data provides a high degree of assurance of the manufacturing of these tablets’ products meet its predetermined specification and quality attributes. Metformin hydrochloride solution of about 100 ppm was accurately prepared for determination of wavelength of maximum absorption. The absorbance of this solution was scanned and measured as a function of wavelength in the 200-400 nm UV regions. The maximum absorption wavelength (λmax) was observed at 234nm, and this wavelength was adopted for absorbance measurement. All spectrophotometer measurements were at room temperature laboratory standard.
The authors are highly thankful to the Faculty of Pharmacy, AIMST University, Bedong, Kedah D.A., Malaysia for funding and providing facilities to carry out this research project.
Authors declare that there is no conflict of interests.


