Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Ozan Emre Eyupoglu1*, Ahsen Isik2, Beyza Kapanci2, Ece Acar2, Elanur Taban2, Esma Onder2 and Miray Bahadir2
Received: September 14, 2022; Published: September 22, 2022
*Corresponding author: Ozan Emre Eyupoglu, Kavacik South Campus, Ataturk Street, Goztepe District, Istanbul Medipol University, School of Pharmacy, Biochemistry Department, 34810, Beykoz/ Turkey
DOI: 10.26717/BJSTR.2022.46.007338
SMA is a rare autosomal recessive neuromuscular junction denervation disease that causes progressive skeletal muscle weakness. Innovative SMA treatments have altered the disease’s trajectory. SMA is an inherited condition that causes child mortality and is the most frequent hereditary disease. There is yet to be discovered a therapy procedure that can completely eradicate this condition. Medical nutrition therapy is one of the most common supportive treatment options for people with SMA. SMA disease can be treated using cell replacement therapy, which is one of the treatment options. The medications utilized in the treatment are some of the most expensive on the market.
Keywords: SMA; Treatment; FDA; Drugs
Abbreviations: SMA: Spinal Muscular Atrophy; SMN 1: Survival Motor Neuron 1; FDA: Food and Drug Administration; SMN 2: Survival Motor Neuron 2; BIPAP: Bi-Level Positive Airway Pressure; HD: Homozygous Deletion; HZD: Heterozygous Deletion; HDAC: Histone Deacetylase; CFS: Cerebrospinal Fluid; CNS: Central Nervous System; FMO: Flavin Monooxygenase; CYP: Cytochrome p450; EphMRA: European Pharmaceutical Market Research Association; HFMSE: Hammersmith Functional Engine Scale; ALT: Alanine Aminotransferase; AST: Aspartate Aminotransferase; ASO: Antistreptolysin
It’s also the most common inherited cause of child mortality. SMA is caused by homozygous omission or loss-of-function mutations in the gene SMN1 (Survival Motor Neuron 1) [1]. Age of onset, 0-6 months Type I (Werdnig- Hoffmann) SMA is the strongest and most severe stage, generally leading to death before 2 times [1]. Type II SMA patients are moderately affected, with age before 18 months [1]. Type II patients cannot walk alone, and the course of the disease is slower [1]. Type III (Kugelberg-Walender) patients can walk without support [1]. It was found to have a milder phenotype than the other groups and the age of onset is after 18 months. Type IV SMA occurs during adulthood. The onset and progression of the disease are slow compared to other types, and even pauses in the progression of the disease can be seen. Adult SMA patients are usually able to walk, but some patients need a wheelchair. Respiratory functions and nutrition are the main subjects of these treatments, but many methods continue to be developed with the developing technology [1]. For this reason, the main purpose of the method to be followed to treat the disease; is to increase the level of SMN (Survival Motor Neuron Protein) in motor neurons with small molecules, oligonucleotides, and gene replacement [1]. However, among the known treatment methods, there are also stem cell studies and approved drugs [1]. Although treatments have advanced in recent times, it has been realized that early opinion has an important part in the effectiveness of medicines. The FDA (U.S. Food and Drug Administration) has approved three new medicines for the treatment of SMA. These medicines are nusinersen (Oligonucleotide), onasemnogene abeparvovec-xioi (Gene Relief), and risdiplam (SMN2 Gene Modulator) [1]. The medicines mentioned in our composition are examined and explained with their pharmacological parcels.
SMA is an inherited disease and the most common inherited disease that cause child mortality. A treatment method that can eliminate this disease has not been found yet. Until recently, supportive treatment methods have been used. These supportive treatments are respiratory functions and nutrition. Many methods continue to be developed with the developing technology and accumulated knowledge experience. The disease, known as SMA, is known primarily to be caused by mutations and deletions in the survival motor neuron gene. Therefore, the main purpose of the method to be followed to treat the disease; is to increase the level of SMN in motor neurons with small molecules, oligonucleotides, and gene replacement [1]. However, among the known treatment methods, there are also stem cell studies and approved drugs. We will examine the treatment methods in more detail at this stage. Medical nutrition therapy is one of the leading supportive treatment methods in individuals with SMA disease. Nutritional problems, which are found to exist at every level of the disease, affect the disease process extremely negatively. While this treatment method positively affects the treatment process of individuals with the disease, it also helps to regulate respiratory functions. This treatment method includes many stages. Taking detailed anamnesis, evaluation of anthropometric, chemical-laboratory findings, calculation of energy and nutritional requirements, selection and implementation of the appropriate diet, close followup, and evaluation are among these stages.
In this treatment method, we should not forget that the energy requirements, growth, and development rates of children with this disease are different from those of healthy children. Considering this, it is necessary to calculate the requirements with special formulas developed for treatment. Otherwise, undesirable results may occur. It has been determined that there is an important relationship between upper extremity functions and respiratory functions of individuals with neuromuscular disease. Respiratory involvement is especially important in types 1 and 2 and should be followed closely, breathing studies should be performed during sleep. When necessary, home-use devices such as BiPAP (Bi-Level Positive Airway Pressure) should be provided [1].
To increase the life expectancy and quality of life of individuals with neuromuscular disease, respiratory functions, and upper extremity functions should be evaluated holistically. Therefore, it is thought that when physiotherapists create a rehabilitation program for these patients, incorporating exercises for the upper extremity, which are often neglected, into the program, along with breathing exercises and training, will contribute to the treatment process. SMN gene therapy is also one of the most promising treatment modalities for SMA patients. Attempts are being made to treat it by giving motor neurons the SMN gene. Methods such as increasing the SMN protein by modifying the SMN2 gene or other genes are the methods used in the treatment of the disease. The aim here is either to increase the number of SMN2 or to make the SMN2 gene make more normal-sized proteins [1].
The knowledge that the increase in the number of copies of the SMN2 gene reduces the severity of the disease in individuals with this disease has guided studies on treatment. It is aimed to increase the level of functional protein by increasing SMN2 gene expression and/or ensuring correct splicing. For this purpose, studies are carried out to modify the structures of known inhibitors and to develop new inhibitors with high inhibition activity and non-toxic effects on cells [2]. According to the results of the research, HDAC (Histone Deacetylase) inhibitors are thought to be more promising. Cell replacement therapy is one of the treatment methods that can be used in SMA disease. It is not yet common in spinal muscular atrophy compared to other methods. The development of effective stem cell therapies for SMA; For maximum therapeutic benefit, it depends on overcoming the difficulties of establishing a functional synaptic connection and replacing an adequate number of motor neurons with the right timing [2]. There are approved drugs used to treat SMA disease. We know that there are three drugs approved by the FDA today.
The first therapy was approved by the FDA in 2016 for the treatment of SMA [2]. Spinraza is an SMN-boosting therapy that works by targeting the SMN2 gene, which makes less protein. It can be used in the treatment of all ages and all types of SMA. Treatment is started with four loading doses in the first two months. It is then continued with a maintenance dose every four months. It is a very expensive drug [3]. According to the results of the research, better results are obtained in the treatment of people who start using this drug immediately after the diagnosis. The drug’s pharmacological properties are given in the table below (Table 1). Nusinersen drug therapy is preferred in SMA treatments in Turkey, as there is no opportunity to participate in gene therapy drug trials in our country. Nusinersen drug therapy has been observed to be beneficial in all types of SMA. Nusinersen drug therapy has been proven to improve motor skills studies [4].
This product is an AAV virus-based gene therapy. It tries to solve the root cause of the disease. It was approved by the FDA in 2019 as the first gene replacement therapy for children under 2 years of age with SMA type I disease [5]. As a result of intravenous administration of zolgensma, SMN protein is produced in the patient’s body, thus muscle movements are preserved. The weight variation of the patient is taken under consideration once determinative the dose of the drug. As a result of clinical trials, it has been proven that zolgensma is the most effective and safe, and that neuromotor steps are improved in patients using this drug. The drug’s pharmacological properties are shown in the table below (Table 2). As seen in the figure above (Figure 1), patients between 2.5 and 3 kilos were given a dose of 16.5 mL. A dose of 19.3 mL was given in the range of 3 to 3.5 kilos [5].
Risdiplam was approved in 2020 for all types of SMA patients older than 2 months [6]. It was the third treatment for SMA to be approved (Table 3). It is in syrup form. It is administered orally every day. Thanks to the drug, it was observed that SMN protein increased both in the spinal fluid and in the surrounding tissues. Although it is cheaper compared to other drugs, it is still a costly drug. Drug doses are determined according to age and body weight. It is not yet available in Turkey [6]. The drug’s pharmacological properties are shown in the table below (Table 4).
When we take all these into account, we can say that progress has been made in the treatment of SMA with the developing technology and the advancing knowledge in recent years. New therapeutic targets such as stem cell applications are currently being studied, through FDA-approved nusinersen (ASO), onasemnogene abeparvovec-xioi (Gene Therapy), and risdiplam (SMN2 Gene Modulator). Early diagnosis is vital in the treatment of SMA. Due to the high cost of drugs, health insurance in some countries can cover the costs of treatment. Onasemnogene abeparvovec-xioi and risdiplam, which are approved drugs, are not currently available in our country. Nusinersen is currently used in Turkey to treat SMA. With the introduction of these new drugs, the clinical phenotype of SMA has improved compared to its natural history, and a new phenotype called curative SMA type 1 has been hypothesized. However, it is important to remember that responses to these therapies may vary in clinical decision-making and family conversations, depending on several factors. Monitoring short- and long-term clinical outcomes is critical as it is too early to see lasting improvements in pulmonary outcomes. Airway aspiration, SDB and hypoventilation, mild cough, and mucociliary clearance remain concerns and require ongoing monitoring until more prospective data are available. These new drugs are most effective when given early, along with preventive multidisciplinary treatment of medical problems. Early treatment of presymptomatic patients had the best results for all three regimens and demonstrated the value of early detection. The impact of newborn screening on early detection and treatment is enormous and has the potential to significantly alter the course and outcome of respiratory disease. Another country where nusinersen is used for therapeutic purposes in France.
In France, SMA cases were treated using nusinersen for one time, and at the end of this one time, the cases’ conditions were observed and turned into statistical data. Nusinersen, a new drug for patients with SMA, has been available in France since May 2017 [7]. Nusinersen is an antisense oligonucleotide that acts as a splicing regulator by repeatedly injecting the SMN2 intron to reach the intronic splicing silencer N1 [7]. Nusinersen has demonstrated some remedial benefits, including long-term survival above 2 times of age, through well-controlled clinical trials in different SMA case populations. However, the rising use of invasive treatments like gastrostomy and respiratory support appears to be neutralizing these motor advances. In the France network for unusual French systems, 23 central controls were handed for SMA cases with rare pediatrics [7]. The neuromuscular commissions of French Pediatric Neurology, whose yearly meetings are held, commissioned this multi-user study. The data for this study was gathered between May 2017 and February 2019 (7). (When nusinersen came available in France) [7]. As seen in the figure above, the number of SMA patients treated with nusinersen is two hundred and four. The number of scanned files is one hundred eighty-nine. Fifteen patients were excluded from the SMA type three groups. In the scanned files, 189 patients were divided into groups among themselves. First group: insufficient data (Under-Evaluation at The Start of Treatment) 13 individuals, second group: nusinersen ongoing, but treatment expected to last less than ten months 48 individuals, third group: Number of patients who died before completing ten months of treatment 5 individuals, and final group cases in final analysis 123 classified as a person.
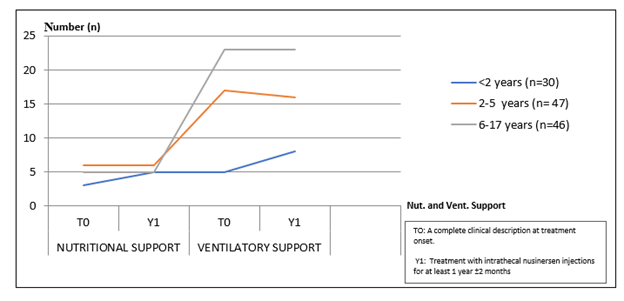
The standard deviation of the incidence of SMA disease was calculated using the Excel Windows 10 version (Figure 2). One patient died of viral cardiomyopathy after 14 months of treatment (Figure 3) [7]. Overall, there was no significant difference in the number of cases receiving respiratory or nutritional support between T0 and Y1, but there was no significant increase in the number of cases receiving respiratory support in younger cases (SMA Type 1) those receiving nutritional support (3 cases in T0, 5 cases in Y1), and those receiving respiratory support (three cases in T0 and five cases in Y1) (five cases at birth) (Figure 2). [7]. Grounded on caregiver assessments of treatment benefits after one time, no caregivers rated cases’ condition as a minimum, significant, or significantly worse for the 105 cases. 95 adverse events were reported in 25 cases [7]. In addition to specialized difficulties related to lumbar perforation in 55 cases, the need for fluoroscopic guidance in 17 cases, headache in 23 cases, postlumbar perforation pattern in 6 cases, nausea and puking in 4 cases, delicacy in 4 cases, back pain in 2 cases, and fever were observed in 1 case [7]. Analogous to other studies, no serious adverse events were observed in this nusinersen study. This study shows that nusinersen improves motor function. After one therapy, all but three (27/30) of the children under the age of two in this trial had significantly improved HINE- 2 scores [7]. However, despite the gains in motor function, none of the children were fit to walk, and the maximum obtained motor position is the same as that of a case with type 2 SMA [7]. Furthermore, the findings indicate that the usage of feeding and respiratory support is increasing. 85 percent of young toddlers are starting to have the ability to undertake maximum diurnal training (eating alone, digging their hair, rolling over during sleep) [7]. Despite this, none of the youngsters were able to walk. Despite these advancements, patients with SMA type 1 and 2 who are treated with nusinersen still require a great deal of probative care and are severely disabled. Children with SMA who admit new treatments have a longer life expectation and an advanced quality of life, but the long-term goods are uncertain and should be nearly covered. Speech and the capability to handle particulars are two important aspects of a child’s development that cannot be explored using broad criteria. Other important rudiments for children, similar as autonomy, body image, and family ties, may be overlooked in standard assessments like the Pediatric Quality of Life tool. The effect of nusinersen on adult quality of life is less well given (Figures 4-6).
Figure 4: Clinical Classification of SMA Subtypes by Onset, Milestones Reached, and Clinical Presentation. Typical Assigned SMN2 Copy Numbers are Shown [4].

Note: Nutrition and Respiratory Support Needs
Figure 6: Number of Patients Requiring Nutritional and Ventilatory Support at T0 and Y1 [7].
While the motor development of grown-ups with SMA is limited, stabilizing motor function and adding energy and stamina can enable cases to keep working, connect with loved ones, and achieve their empirical and interpersonal pretensions. Increased energy situations allow cases to stand longer during the day and perform better at work or academy, maintain their voice and speech capacities, write longer, spend further time in front of a computer without fatigue, and spend further time on pillars rather than a wheelchair. these are all exemplifications of clinically applicable treatment benefits not covered by complaint standing scales. Using being measures, it’s also problematic to quantify the consequences of reduced educational openings or low social integration on cases’ quality of life. More importantly, SMA cases impact the healthrelated quality of life of their families, caregivers, and the public. Cases may be fully dependent on their caregivers. For illustration, parents and family may be sleep deprived because of having to turn their baby over during the night.
This study includes research on the types of SMA disease, treatment processes, treatment costs, and the drugs accepted and used in the treatment process. Today, there are three FDA-approved drugs, these are nusinersen (ASO), onasemnogene abeparvovecxioi (Gene Therapy), and risdiplam (SMN2 Gene Modulator). In addition, studies on new treatments such as stem cell applications continue. Treatments that can improve the vital functions and protect the health of SMA patients are an important clinical imperative. Therefore, early diagnosis is of great importance for SMA disease. According to the information we have obtained from our research, the most used drug is nusinersen (ASO). It has been proven in studies that this drug improves the motor skills of SMA patients, but no cure has been achieved because any treatment. According to the information we have obtained from studies conducted in Turkey and France, it has been observed that the motor skills of SMA patients have improved. Other FDA-approved drugs such as onasemnogene, abeparvovec- xioi, and risdiplam are not currently used in Turkey. The medicines used in the treatment process are among the most precious medicines in the world. The drugs named onasemnogene, and abeparvovec-xioi (zolgensma) are the most effective and definitive drugs used in the treatment of SMA disease, but they are difficult to reach due to their cost.
This study was written in the light of the early diagnosis and treatment of SMA disease to become a priority in the coming years.


