Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Arshad Khan*
Received: September 15, 2022; Published: September 21, 2022
*Corresponding author: Arshad Khan, Chemistry Dept, Pennsylvania State University, DuBois, PA 15801, USA
DOI: 10.26717/BJSTR.2022.46.007334
For a dilute solution (0.15 mg/L) of α-amylase, substantial inactivation takes place when just 0.1% caffeine is added at room temperature (25°C). Interestingly, the amount of inactivation remains almost unchanged by the additional amount of caffeine. For a 3X more concentrated solution of enzyme, almost no inactivation is observed even when the caffeine concentration is increased to around 0.3%. Herein, we explain why only the dilute enzyme solution is inactivated by caffeine and secondly, why only the dilute caffeine solution causes protein inactivation. The inactivation of the enzyme means a reduced amount of starch hydrolysis by this enzyme and hence, a smaller amount of sugar formation. This finding may indicate health benefits of caffeine, especially to those having issues with blood sugar.
α-Amylase is a metallo-enzyme that can be obtained from bacterial (bacillus), fungal or various animal sources. Irrespective of sources, they exhibit similar [1-7] properties like hydrolysis of starch molecules to sugar units and the inactivation of the protein caused by heat and other additives [8-11]. The inactivation model[8] for α-amylase comprises a reversible stage (eq 1) that forms an inactive apoenzyme, E2-, from the active enzyme, CaE, with a rate constant of k1 and a reverse reactivation reaction with a rate constant of k-1 followed by an irreversible (eq 2) reaction that forms a denatured enzyme, EI2-, with a rate constant of k2.
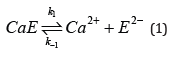
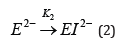
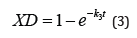
where, 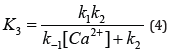
The expression XD (eq 3) gives the fraction of enzyme inactivated at a time, t, and the percentage of the active enzyme is given by (1-XD) 100. A detailed derivation of the fraction of enzyme inactivated (XD) has been presented in reference 8.
Recently we reported studies on the effect of caffeine on α-amylase activity [10] and presented these results in Figure 1. The bottom curve (blue) of Figure 1 represents the most diluted enzyme (0.15 mg/L) from bacillus species and shows a large inactivation (by 50%) due to the addition of a small amount of caffeine (0.1%). Additional amount of caffeine up to 0.3% does not show any significant change in protein activity. The third curve from the bottom (pink) represents 2X concentrated (0.30 mg/L) enzyme (bacillus) and shows a much smaller inactivation (by 30%) even after the addition of 0.3% caffeine. The fourth curve from the bottom represents 3X more concentrated enzyme (0.45 mg/L) and shows only 10% inactivation by caffeine of 0.1-0.3% concentration. The second curve from the bottom represents human enzyme with a concentration of 0.25 mg/L and shows a sharp decrease in activity after the addition of 0.1% caffeine. There are two key findings in these studies; one, diluted enzymes are most vulnerable to inactivation by caffeine and secondly, the diluted caffeine solutions show the maximum inactivation of the protein (0.05-0.1%) in comparison with the more concentrated ones.
Here we propose a cluster model for the enzyme. As enzyme concentration is increased, a number of protein molecules bind together and form clusters, which are resistant to inactivation.
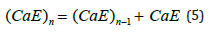
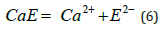
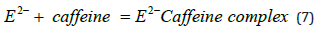
The left-hand side of eq (5) shows how the n molecules of the enzyme are bound together to form clusters. It is assumed that the cluster molecules, (CaE)n, are in equilibrium with smaller clusters, (CaE)n-1 and unassociated enzyme molecules, CaE that undergo inactivation. From an earlier experiment, we know that high concentrations of the enzyme solutions are resistant to inactivation by heat or EDTA [8]. Here we also see that such a solution is also resistant to inactivation by caffeine. These results are consistent with the cluster model presented here. In relatively concentrated solutions, when most enzyme molecules are involved in cluster formation, only a few are available unassociated to undergo inactivation reactions shown in eqns 6 &7. Because of the presence of a low concentration of the unassociated enzyme, the inactivation rate due to caffeine drops. To understand why only the diluted caffeine solution (0.05-0.1%) works the best for inactivation, we can suggest two possible reasons; one, in concentrated caffeine solutions, some of the molecules are associated together forming caffeine binary structures [12,13] and in diluted solutions unassociated caffeine molecules (Figure 2) are available which can presumably cause disruption of the H-bond of the protein structure (Figure 2) resulting in inactivation. The top of Figure 2b shows the helical structure of protein with its H-bonds and the bottom figure of 2b shows pleated sheet with H-bonds holding two protein chains together. To break such an H-bonding structure, the caffeine molecules need to have sufficient energy (activation energy) to break the C=O ….H-N.. bond of protein and form a new bond with caffeine’s C=O bond (Figure 2). It is quite likely that at room temperature (25°C) only a limited number of such bond breaking can happen causing levelling off of the enzyme inactivation by caffeine.


