Abstract
Introduction: Preterm delivery is defined as a birth that occurs before 37 completed weeks or 259 days of gestation. One of the most common morbidities associated with prematurity is respiratory distress syndrome (RDS), which is manifested by progressive respiratory failure shortly after birth. RDS is a syndrome, affecting premature neonates, resulting from developmental insufficiency of surfactant production coupled with structural immaturity of the lungs. Surfactant decreases alveolar surface tension, and its absence would be expected to render the immature lung less compliant. One method to determine fetal lung maturity (FLM) and the ability to produce sufficient surfactant is lamellar body count (LBC). Lamellar bodies are a byproduct of surfactant, which is found in the amniotic fluid after 20 weeks of gestation. LBC is performed using a simple blood count analyzer; therefore, it is inexpensive, readily available, and does not require laboratory technicians to receive any special training.
Objective: The aim of this study is to determine whether the LBC test performed from a vaginal swab, following preterm premature rapture of the membranes (PPROM) could be used to predict fetal lung maturity and to assess the risk of RDS.
Methods: a retrospective cohort study comparing the LBC index in amniotic fluids of samples taken from vaginal swabs of women in their 28-36th week of a singleton pregnancy and the prevalence of RDS in the neonate.
Results: In the data collected we observed an LBC range from 1 to 370 with an average of 40.9. when the LBC was conducted at an earlier stage of gestation the LBC was higher.
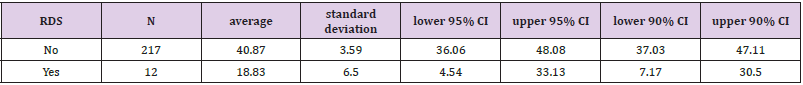
a significantly lower LBC (p = 0.005) was found in cases with RDS (18.8 3± 6.5) compared to cases without RDS.
Cases of RDS were born earlier, had the lower LBC scores, and higher incidence of jaundice and IUGR compared to cases without RDS.
Conclusion: We found a significant association between low LBC and RDS. LBC lower than 33 is a risk factor for RDS.
Keywords: Osteosynthesis; Cerclage Cable; UHMWPE Cable; Optimal Clamping Force; Contact Pressure
Abbreviations: RDS: Respiratory Distress Syndrome; FLM: Fetal Lung Maturity; LBC: Lamellar Body Count; PPROM: Preterm Premature Rapture Of The Membranes; NEC: Necrotizing Enterocolitis; PG: Phosphatidylglycerol; ACOG: Accepted Guidelines
Introduction
Preterm delivery is defined as a birth that occurs before 37 completed weeks or 259 days of gestation. It is associated with about a third of infant mortality in the Western world. As the gestational age drops, the infant is more susceptible to premature morbidity, hypothermia, respiratory distress syndrome (RDS), cerebral hemorrhage and necrotizing enterocolitis (NEC) among other problems. The prominent and leading cause for premature mortality is RDS. RDS is characterized by respiratory failure requiring artificial respiration, in conjunction with characteristic signs on a chest radiograph [1]. It is known that the reason for this condition is the immaturity of pneumocytes and lack of surfactant, which decreases the surface tension of pulmonary alveoli (normal surface tension of the alveoli would allow for better pulmonary compliance). In other words, for the fetus to be able to breathe independently and efficiently, the pulmonary alveoli cells must be mature enough to create surfactant [2]. The necessity of evaluating the degree of lung maturity of the fetus before birth is debated. Some argue against evaluating prenatal lung maturity because it is not the only important system, and because physicians should strive to prolong the pregnancy as close as possible to the estimated delivery date regardless of this evaluation. Proponents of prenatal evaluation of FLM argue that an immature respiratory system is associated with significant morbidity and mortality. Assessing maturity is increasingly more important in cases of preterm premature rupture of the membranes (PPROM) where the decision is between delivering a very preterm infant or extending the pregnancy and risking infection.
There are several common methods for assessing fetal lung maturity. Lecithin sphingomyelin ratio (L/S ratio), is the oldest method. It is an invasive test (requiring amniocentesis) which requires skill and has limited predictive value in situations such as diabetes. Furthermore, the test is performed using expensive and not readily available machinery - Thin layer chromatography - which requires detailed planning and employee training. Results of this test are not available for about 24 hours and inappropriate storage or preparation of the test drastically affects the results. The advantage of this method is that it is probably not influenced by the presence of meconium-stained or bloody amniotic fluid. Another available test is the detection of phosphatidylglycerol (PG), which is also a byproduct of mature lungs and surfactant production. However, PG is present in the amniotic fluid only from week 35. Therefore, this test has very limited utility. Although the presence of PG almost completely excludes the possibility of RDS, its absence is not informative. In addition, like L/S ratio measurement, the PG test results may also vary when taken using a vaginal swab due to bacterial contamination and therefore PG testing requires amniocentesis [3]. This study focused on the Lamellar Body Count (LBC) method which counts lamellar bodies, a by-product of surfactant in the lungs, with a hematologic analyzers used for a standard blood count. Lamellar bodies are the same size and diameter as thrombocytes and therefore, can be counted with equipment found in any hematology laboratory. Thus, this method is affordable and widely accessible, requires no special training, and the results are available within a few minutes. A disadvantage of this method is that samples stained with blood may affect the results.
Lamellar bodies are secretory organelles produced by Type 2 fetal pneumocytes. These organelles contain phospholipids which are major components of surfactant – an essential substance decreasing surface tension on lung alveoli necessary for respiration. In secretion of these organelles surfactant is secreted by exocytosis into the alveolar space, creating a layer of surfactant on the alveoli surfaces. Lamellar bodies appear in the cellular cytoplasm around week 20 of pregnancy, and in amniotic fluid in the following week. A lack of surfactant is known today to be the cause of RDS in neonates. Since surfactant is an essential substance for neonatal respiration, correlation is expected between levels of lamellar bodies in amniotic fluid, the amount of surfactant produced, and fetal lung maturity. Several studies have established the link between the number of lamellar bodies in amniotic fluid and lung maturity of the newborn. Verder et al. examined the lamellar body counts in aspirate from infants born at 30 weeks gestation and found that a cut-off of 8,000 U/μl determined fetal lung maturity with a sensitivity of 75% and specificity of 72% [4]. The study was conducted on 200 infants, at different centers and laboratories, and showed a strong relationship between the number of lamellar bodies and lung maturity. These test results may help the team prepare for the treatment of the newborn and determine whether to administer surfactant (which is not administered to 50% of infants born before week 30 (3)) and prompt treatment with surfactant has been shown to improve the prognosis of the neonate [5].
Walker et al. examined 249 samples of lamellar bodies from women between 37-41 weeks gestation who were delivered by elective cesarean section. Theses samples were taken during surgery. The study found that each additional week of pregnancy increased the LBC, with the highest increase in production during weeks 37-38. This study only examined births that were at term, therefore the variance was not high enough to establish a threshold degree of lung maturity, certainly at the earlier stages of pregnancy, who are characterized by RDS [6]. Joutsi-Korhonen et al. tested the degree of correlation between the L/S ratio and examination of lamellar bodies from amniocentesis using XE-2100 hematological analyzers (Sysmex, Chuo-ku, Japan) and found a good correlation. This work set a threshold value of 35,000 U/μl to determine lung maturity, and 6,000 U/μl to determine immaturity with a large intermediate area of 7000-35000 U/μl. Therefore, it was recommended to use the test as primary screening, and use the thin layer chromatography method for the intermediate values. [7] However, this study considered only samples in cases of IUGR and macrosomia in the setting of possible gestational diabetes, and 6 samples from twin pregnancies. This may have influenced the variance in lamellar body counts and made the identification of a clear threshold for review more difficult. In addition, the study noted that diabetic women had significantly more lamellar bodies than non-diabetic women did, even though one of the known complications of neonates of diabetic mothers is RDS.
This is consistent with other studies on diabetic women [8]. This matter raises the question of whether the RDS mechanism in these infants is not producing enough surfactant, or whether is it somehow deficient qualitatively. it is possible that a higher threshold might be required for these women, but this is beyond the scope of this study. In addition, in the study, 69% of the women were examined at 37-40 weeks of gestation. It is possible that the difference in the number of lamellar bodies during this period is negligible in accordance with the advanced fetal lung maturity. Besnard et AL published a meta analysis comparing the LBC test and the L/S ratio in predicting fetal lung maturity. The study included 13 studies conducted between 1999-2009 in which lung maturity was examined using both methods and produced ROC curves for each. The results showed that testing the lamellar bodies was as least as effective as the L/S ratio and can be used as a test for the majority and not only for initial screening. However, in some of the studies included, it was not specified whether some women had diabetes, whether it was a multiple pregnancy and other factors that could have affected the count and the outcome. Although the aim of this study was to compare standard testing methods and differs from the purpose of the present study, it adds to evidence that the lamellar body test is safe and worthwhile [9].
One study investigated determining lung maturity prematurely and gathering amniotic fluid from the surface of the vagina following PPROM. Salim et al collected vaginal samples of amniotic fluid for testing LBC from 75 women presenting with PPROM who were 27 to 36 weeks pregnant. This study determined that over 28,000 U/μl indicates fetal lung maturity with a specificity of 100% and sensitivity of 75% and that below 8,000 U/μl fetal lungs are not mature, with a sensitivity of 98%. This study includes a large intermediate area, and we believe that there were insufficient samples to reach a more precise threshold [10]. We found only one study that claimed testing LBC using an amniotic fluid sample from the vaginal surface was not effective. This retrospective study from the Netherlands, compared the assessment of lung maturity by amniocentesis and tested the L/S ratio and the lung maturity by checking LBC vaginal swabs after PPROM. The L/S ratio group included 260 maternity patients and the LBC group included 76 women in labor. The study found the LBC testing was inferior to the usual examination and is unsuccessful in predicting RDS. In this study, the ROC curve of the LBC from vaginal samples for predicting RDS appears almost random. However, the study had several weaknesses: first, testing the LBC occurred in different centers by various devices, while it has been clearly shown in previous studies that this leads to varying results and a more stringent threshold is needed for some of these devices [11]. in this study a value of 20,000 U/μl LB was chosen as the threshold for predicting fetal lung maturity, but it was not specified how this value was chosen, and it may be too low.
The issue of the management of birth in the case of PPROM is debated in the literature. Preterm rupture of membranes and conservative management may lead to infection, whereas early, active delivery endangers the neonate with complications related to prematurity. The PPROMT study addressed precisely this dilemma. The study showed that induction of labor in women with PPROM between 34-36 weeks without obstetrical indications, suffered various complications of prematurity, including RDS. It is possible that for these cases, the vaginal surface LBC test will help determine when to schedule the delivery date when the risk of RDS is minimal, before the development of an intrauterine infection endangers the mother and the fetus. Currently, the LBC test is the most promising emerging test for determining fetal lung maturity. It is inexpensive, accessible, sensitive, and several studies have shown that it can predict lung maturity based on samples from vaginal swabs taken following premature rupture of membranes. The aim of this study is to examine if LBC taken from vaginal swab after PPROM, can predict fetal lung maturity (FLM) and the risk of RDS.
Methods
This retrospective cohort study included 307 pregnant women in the second and third trimesters of gestation. vaginal samples of amniotic fluid after PPROM were taken from these women and the LBC was examined, in addition to the 307 babies born to these women. Inclusion criteria for the study population were pregnant women at 28-41 weeks of gestation with PPROM with a singleton pregnancy and no need for immediate delivery, such as chorioamnionitis or fetal distress. Exclusion criteria for the study population were multiple pregnancies, fetuses without a pulse, longer than 14 days between the collection of the sample and delivery, bloody samples (the machine counts platelets as lamellar bodies), and women who arrived at the emergency room with ruptured membranes and immediate indication for delivery- such as chorioamnionitis or fetal distress. Data collected from electronic medical records included maternal demographics (maternal age, gestational diabetes, pre-eclampsia) pregnancy follow-up (fetal sex, intrauterine growth restriction (IUGR), gestational age at delivery, and pregnancy complications at birth i.e pre-eclampsia, gestational diabetes etc.). Delivery data - type (spontaneous vaginal birth, birth by instruments or cesarean), maternal fever during delivery, indication of complications during labor and delivery (nonreassuring monitoring, meconium). Data obtained on newborns included outcomes of RDS according to the accepted clinical definition: respiratory failure of a premature neonate (including the need for mechanical ventilation, surfactant and admission to the neonatal intensive care unit) near delivery, combined with a typical chest x-ray [12] as noted in the medical records. Neonatal data included sex, birth weight, Apgar scores, umbilical artery blood gas values and jaundice.
The Research Variables
The samples were collected using a vaginal swab following rupture of membranes in the delivery room and in the high-risk pregnancy department at Meir Medical Center. The samples were taken and the count was carried out on blood counts analytic device type ADVIA 2120i within a few hours. The information was saved in the BIRTH CARE system and the OFEK system The women’s Data was taken from the BIRTH CARE system (medical acceptance letters, birth report, financial analysis emperors, letters of release) and the data of the neonates were taken from METAVISION and OFEK. The data was collected in tables comparing the index of the lamellar bodies and the presence of RDS in the newborn.
Statistical Methods
To test the hypothesis, comparison was made between the index of the lamellar bodies found in the samples obtained from the women and the outcome of the neonates. In order To examine the differences between the Variables, a t-test for two independent variables was used. To examine differences in distribution between groups, chi-square tests were performed. Finally, to examine the contribution of the independent variable (lamellar bodies) on the dependent variable (neonatal respiratory complications), logistic regression was conducted taking into consideration potential confounders. During Regression analysis stratification by week of pregnancy was made.
Sample Size
In calculating the sample size, we assumed that the incidence of RDS in the neonates (mostly premature babies) in our study will be approximately 20%, consistent with data previously reported in medical literature [13]. According to this assumption, the sample size was based on a proportion confidence interval using the formula:

z for the confidence interval being 1.96, e (acceptable deviation) being 0.05, and the proportion P being 0.2. The sample size obtained was 246 with a anticipation of 50 cases of RDS. Since our ratio was stringent, we expected a higher rate of newborns suffering from RDS. In this scenario, it will be possible to characterize risk factors and prediction variables for those neonates, mainly the LBC.
Results
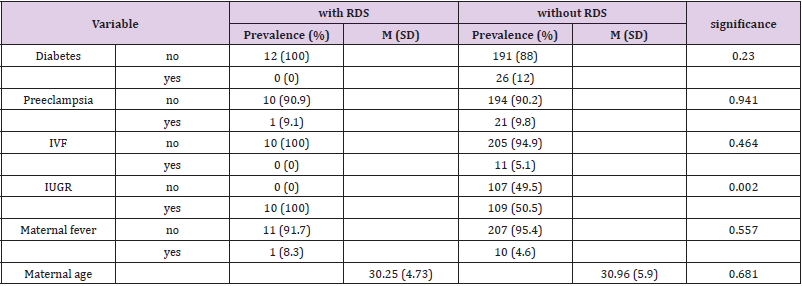
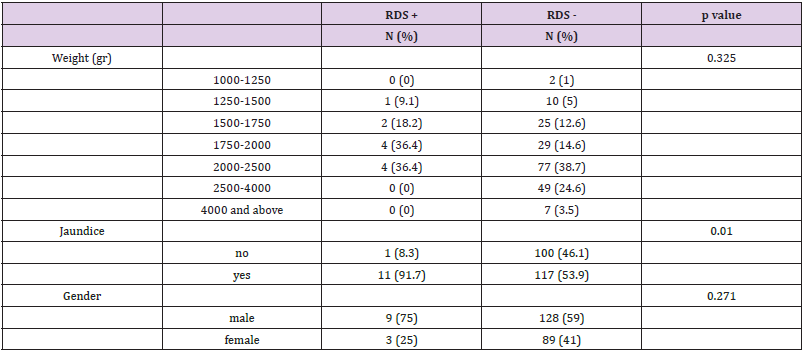
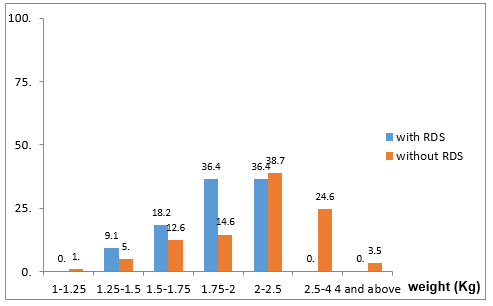
The study included 307 samples taken from vaginal swabs of women following rupture of membranes. Seventy-eight cases did not meet the study inclusion criteria: 38 cases delivered babies more than two weeks after the sample was taken, 35 cases were multiple pregnancies, two cases were stillbirths and the sample was contaminated in three cases. A total of 229 cases met the inclusion criteria and were included in the study. To examine differences between neonates with and without RDS, chi-square tests, t-tests for independent samples, and Kruskal-Wallace tests for independent samples were used. Maternal clinical and demographic data of the study are presented in Table 1. The age range of the mothers ranged from 17 to 55 years, with an average of 30.9 (SD 5.8) years. Gestational diabetes was present in 11.4% of the mothers and 9.7% had preeclampsia. Neonatal data is presented in Table 2. There were 40.2% females and 59.8% males. Gestational age at birth ranged from 31 to 41 weeks, with an average of 34.3 (SD 1.9) weeks. The distribution of the infants’ weights at birth was as follows- 38.6% weighed 2 to 2.5 kg, 23.3% weighed 2.5 to 4 kg, 15.7% had a weight of 1.75 to 2 kg, 12.9% had a weight of 1.5 -1.75 kg, 5.2% had a weight of 1.25 to 1.5 kg, 3.3% weighed 4 kg and above, while 1% weighed 1 to 1.25 kg. In the LBC a range from 1 to 370 was observed with an average of 40.9 (SD 44.2). in terms of IUGR, 52.7% of fetuses shown growth retardation while 47.3% did not. Most (51.1%) were born by Caesarean section, while the rest (48.9%) were vaginally delivered. In addition, 4.9% were conceived through IVF. 4.8% of the mothers had fever. 55.9% of infants had jaundice and 5.2% of infants suffered from RDS. As shown in Table 1, no significant differences in maternal data were observed in both groups (with and without RDS) in the average age, the rate of maternal fever, preeclampsia, diabetes and in vitro fertilization.
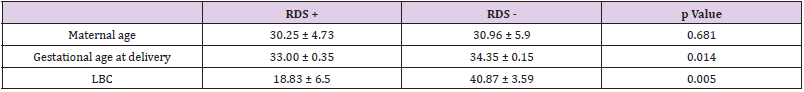
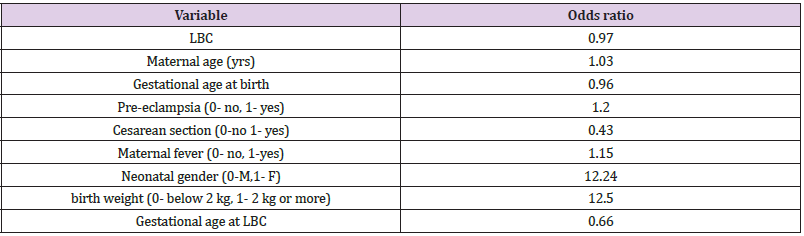
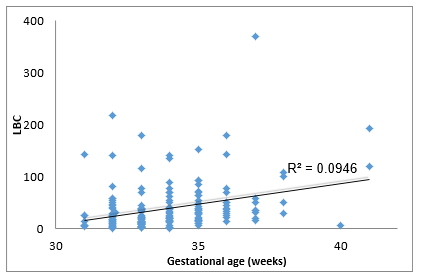
It was found that neonates with IUGR had statistically significant less incidence of RDS (10%) than neonates that were not IUGR (50.5%) (Χ2 = 9.408, p = .002). As shown in Table 2, a significantly lower LBC (p = 0.005) was found in cases with RDS (18.8 3± 6.5) compared to cases without RDS (40.87 ± 3.59). In addition, cases with RDS were delivered earlier (33.00 ± 0.35 weeks) compared to cases without RDS (34.35 ± 0.15) (p = 0.014). in Table 3 it is shown that there were no significant differences between the newborns with or without RDS in gender or weight. however significantly more newborns with RDS had jaundice (91.7%) when compared with newborns without RDS (53.9%). Analysis of the relationship between gestational age and LBC (Figure 1) had a strong positive correlation (r = 0.308, p = 0.000). meaning when the LBC was conducted at an earlier stage of gestation the LBC was higher as demonstrated in Figure 2. To test whether research metrics (including gestational age at LBC) significantly predicted RDS, logistic regression was conducted (Figure 2) with all the indicators in the study confounders. As mentioned above, since there were no cases of RDS among cases with diabetes, IVF, and IUGR, it was not possible to include these measures in the analysis. The regression results are shown in Table 4. The model correctly classified RDS 93.6% of the time, but did not significantly explain differences in RDS (X2 = 15.251, p = 0.084). Thus, none of the confounding factors significantly predicted chance of RDS. In order to examine the relationship between RDS and LBC, the confidence intervals of LBC were tested for infants with RDS and without RDS. Table 5 shows the mean, standard deviation, and confidence intervals that were found.
Table 4: Logistic regression predicting RDS according to research variables.
Note: *P<0.05, **P<0.01.
Main Findings
We found a direct correlation between the occurrence of RDS and LBC, gestational age at birth, IUGR, and jaundice. That is, cases of RDS were born earlier, had the lower LBC scores, and higher incidence of jaundice and IUGR compared to cases without RDS. It can be said that with a 95% confidence interval, the result of LBC higher than 36.06 indicates pulmonary maturity, while LBC ≤33 indicates a risk of RDS.
Discussion
Primary outcomes
The aim of this study was to determine if LBC from amniotic fluid collected from the vaginal surface after PROM can predict neonatal lung maturity and chances of RDS. The issue of determining fetal lung maturity by LBC has been reported in the literature, however very few reports examined swabs collected from the vaginal surface after PROM. Moreover, in literature and accepted guidelines (ACOG) there are acceptable thresholds for pulmonary maturity (50,000 units) and thresholds for pulmonary immaturity (15000 units) with a large interval remaining that does not aid in medical team decision making. LBC levels obtained in the early weeks of pregnancy and from infants with RDS are significantly lower than those obtained in later weeks of pregnancy and healthy infants. In this study, performed on 307 samples taken from vaginal surfaces at Meir Hospital, a clear correlation was found between the LBC result and the risk of RDS. Levels of LBC from newborns with RDS are significantly lower (p = 0.14) than the count in healthy neonates. the average result of the LBC among infants with RDS was of 18.83± 6.5, while the average result of the LBC among the group of healthy newborns was 40.87± 3.59. These results correspond to previous studies conducted from samples taken from amniotic fluid during amniocentesis [14] and in fact show that the sample obtained via a vaginal surface swab is not inferior to a sample taken during amniocentesis assuming the sample is not bloody.
In addition, there are significant differences (p = 0.005) between the groups regarding gestational age. as expected, at earlier gestational ages, fetal lung maturity is lower and there are more cases of RDS. Among the group of neonates with RDS, the average week of pregnancy was 33 (with a standard deviation of 0.35) while in the healthy neonates, the average was 34.35 (SD = 0.15). This average is in accordance with previous studies [13,14] and moreover, it validates the logic of treating with injections of celestone for fetal lung maturity up to 34 weeks and not after. This study found a new threshold value for determining lung maturity: in an LBC test obtained from the vaginal surface, with a 95% confidence interval, an LBC greater then 36.06 indicates pulmonary maturity, while a value of 33 or less indicates a risk of RDS. The intermediate values from the ACOG guidelines were narrowed down considerably.
Secondary Outcomes
Another interesting statistic from this study is the difference between the weight of the newborn and RDS. The logistic regression test distinguished between infants of the same birth weight, but with different values for RDS (yes/no). In our study, it seems that there is no connection whatsoever between the birth weight of the newborn alone and the chances of developing respiratory distress syndrome, but there is a definite link between IUGR (newborn weight less than 10% by weight appropriate for gestational age) and RDS. That is, IUGR itself, regardless of the absolute weight of the newborn, appears to be a risk factor for RDS. This finding has been noted in previous studies regarding the effects of IUGR on newborns [15]. It is possible that reduced renal function impairs the normal manufacturing process of surfactant. This issue requires more thorough investigation, and it is not included of our study. Jaundice was also found more often among infants with RDS. This finding also requires more in-depth investigation, because we do not know the causal relationship between these two phenomena. There was no association between maternal age, maternal fever during labor, gender or cesarean delivery and neonatal RDS. It should be noted that as part of our data collection we did not always find the indication for the Caesarean section (whether due to previous Caesarean, fetal lie, maternal distress or other reasons). This issue also requires more careful attention and is not considered in this study.
Strengths and Weaknesses
Strengths of this study are that the LBCs were all performed using the same method, within 24 hours, in the same laboratory and with the same equipment. Thus, the multivariate calibration and material handling were effectively neutralized. However, this study has several weaknesses: the incidence of RDS found in our study was significantly lower than we expected for the calculated sample size (p = 0.2). This assumption was based on the proportion of different reports in the literature regarding the incidence of RDS in premature infants (who formed most of the study population) [13]. Looking back this can be attributed to several biases in information:
1. The exclusion criteria were cases of multiple pregnancies, which are characterized by lower gestational ages and lower lung maturity.
2. Cases of quick, premature births were apparently excluded (it is likely that in such cases no vaginal sample was taken due to the rapid delivery). These births did not have time to receive a complete course of celestone, and most cases are characterized by higher incidence of RDS.
Nevertheless, the incidence of RDS in this study (5.2%) is sufficient for investigating the degree of correlation between the LBC and incidents of RDS and for the examination of other variables evaluated in the study. In addition, this is a small, retrospective study, with all the limitations of this method. We have no information about selection of patients who underwent the test and therefore no way to anticipate and neutralize selection bias. our control of confounding variables is incomplete and we relied on reports in the medical records as they were recorded and available to us.
Limitations- Potential Biases
Selection Bias: All samples were taken at Meir Hospital, which serves the residents of the Sharon and the surrounding area only. It will be possible to overcome this bias by comparing the data to other hospitals in a different location in future research. Another source of selection bias is maternal characteristics. There is inconclusive evidence that women with gestational diabetes have higher LBC values compared to women with normal pregnancies, even though newborns of diabetic women tend to have more complications, including RDS [7]. We overcame this bias by including gestational diabetes in maternal demographic data and stratifying accordingly.
Confounding Information: bloody samples may potentially affect the number of lamellar bodies because platelets may be counted as lamellar bodies by the machine. Therefore, bloody samples were excluded from our study. In addition, it is unknown in the literature whether the presence of meconium in the amniotic fluid may affect c the values of the LBC, so we included these cases in the regression. To account for these biases, we used a multivariate model that was corrected for additional variables such as maternal fever, amnionitis, gestational diabetes, meconium fluid, neonatal fever or diagnosis of neonatal respiratory infection [16-19].
Significance
As noted above, the issue of management of delivery in the case of premature rupture of membranes (PPROM) is debated in the literature. PPROM and conservative management imperils the fetus to vaginal infection on one hand, while on the other hand early active delivery risks complications of prematurity, as noted above. Although there is debate about the necessity of testing fetal lung maturity prior to birth (as some say it is only in one of several systems that need to mature), we believe that the immaturity of the pulmonary system is associated with significant morbidity and mortality and therefore has great significance. We believe that in borderline cases of PPROM in advance pregnancies, as described above, an LBC test from a vaginal swab could help tip the scales as to the timing of delivery. The test is inexpensive, fast, safe, requires no special training, and as we have shown in our study, is reliable and effective in determining the extent of fetal lung maturity. Although most dilemmas regarding delivery are relevant in the later weeks of pregnancy (34-36), and this study examined a much wider range, we believe that it is a starting point for a deeper examination of this issue. Larger, prospective, multicenter randomized studies are required to substantiate this conclusion.
References
- Visnjevac J, Novakov-Mikić A, Nikolić A (2010) The ways of amniotic fluid sampling and its influence on lamellar body count. Med Pregl 63(7-8): 483-486.
- Verder H, Ebbesen F, Brandt J, Dahl M, Esberg G, et al. (2011) Lamellar body counts on gastric aspirates for prediction of respiratory distress syndrome. Acta Paediatr 100(2): 175-180.
- Plauché WC, Faro S, Letellier R (1982) Phosphatidylglycerol and fetal lung maturity. Am J Obstet Gynecol 144(2): 167-172.
- Kendig JW, Notter RH, Cox C, Reubens LJ, Davis JM, et al. (1991) A comparison of surfactant as immediate prophylaxis and as rescue therapy in newborns of less than 30 weeks` gestation. N Engl J Med 324(13): 865-871.
- Egberts J, Brand R, Walti H, Bevilaqua G, Breart G, et al. (1997) Mortality, severe respiratory distress syndrome, and chronic lung disease of the newborn are reduced more after prophylactic than after therapeutic administration of the surfactant Curosurf. Pediatrics 100(1): e4.
- Walker SP, Chow YY, Ugoni AM, Holberton JR, Smith CL, et al. (2010) Amniotic fluid lamellar body concentration as a marker of fetal lung maturity at term elective Caesarean delivery. Aust N Z J Obstet Gynaecol 50(4): 358-362.
- Joutsi-Korhonen L, Aitokallio-Tallberg A, Halmesmäki E, Hämäläinen E (2010) Amniotic lamellar body counts determined with the Sysmex XE-2100 analyzer to predict fetal lung maturity during diabetic and other complicated pregnancies. Scand J Clin Lab Invest 70(5): 358-363.
- Ghidini A, Spong CY, Goodwin K, Pezzullo JC (2002) Optimal thresholds of the lecithin/sphingomyelin ratio and lamellar body count for the prediction of the presence of phosphatidyl glycerol in diabetic women. J Matern Fetal Neonatal Med 12(2): 95-98.
- Besnard AE, Wirjosoekarto SA, Broeze KA, Opmeer BC, Mol BW (2013) Lecithin/sphingomyelin ratio and lamellar body count for fetal lung maturity: a meta-analysis. Eur J Obstet Gynecol Reprod Biol 169(2): 177-183.
- Salim R, Zafran N, Nachum Z, Garmi G, Shalev E (2009) Predicting lung maturity in preterm rupture of membranes via lamellar bodies count from a vaginal pool: a cohort study. Reprod Biol Endocrinol 7(1): 112.
- Szalla A, Gronowski AM, Eby CS (2003) Lamellar Body Count in Amniotic Fluid: A Comparative Study of Four Different Hematology Analyzers. Clin Chem 49(6 Pt 1): 994-997.
- Somaschini M, Nogee LM, Sassi I, Danhaive O, Presi S, et al. (2007) Unexplained neonatal respiratory distress due to congenital surfactant deficiency. J Pediatr 150(6): 649-653.
- Mercer, Brian M (2014) Creasy and Resnik’s Maternal-Fetal Madicine: Principles and Practice (7th)., 42: 663-672.e4.
- Karcher R, Sykes E, Batton D, Uddin Z, Ross G, et al. (2005) Gestational age-specific predicted risk of neonatal respiratory distress syndrome using lamellar body count and surfactant-to-albumin ratio in amniotic fluid. Am J Obstet Gynecol 193(5): 1680-1684.
- Gilbert WN, Danielsen B (2003) Pregnancy outcomes associated with intrauterine growth restriction. Am J Obstet Gynecol 188(6): 1596-1599.
- Lia DE Wijnberger, Madelijn de Kleine, Hieronymus AM Voorbij, Birgit Arabin, Henk Engel, et al. (2010) Comparison of vaginal and transabdominal collection of amniotic fluid for fetal lung maturity tests. J Matern Fetal Neonatal Med 23(7): 613-616.
- Tsuda H, Takahashi Y, Iwagaki S, Kawabata I, Hayakawa H, et al. (2010) Intra-amniotic infection increases amniotic lamellar body count before 34 weeks of gestation. J Matern Fetal Neonatal Med 23(10): 1230-1236.
- Gluck L, Kulovich MV, Borer RC Jr, Keidel WN (1974) The interpretation and significance of the lecithin-sphingomyelin ratio in amniotic fluid. Am J Obstet Gynecol 120(1): 142-155.
- Tabsh KM, Brinkman CR, Bashore R (1982) Lecithin:sphingomyelin ratio in pregnancies complicated by insulin-dependent diabetes mellitus. Obstet Gynecol 59(3): 353-358.

 Research Article
Research Article